|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB002036 |
|---|
|
Identification |
|---|
| Name: |
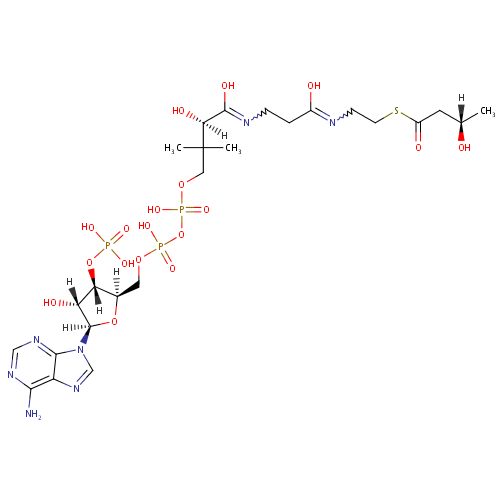
(S)-3-Hydroxybutanoyl-CoA |
|---|
| Description: | (S)-3-hydroxybutanoyl-CoA is a member of the chemical class known as Coenzyme A and Derivatives. These are derivative of vitamin B5 containing a 4'-phosphopantetheine moiety attached to a diphospho-adenosine. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (3S)-3-hydroxybutanoyl-CoA
- (S)-3-Hydroxybutanoyl-CoA
- (S)-3-hydroxybutyroyl-CoA
- (S)-3-hydroxybutyryl-coa
- 3'-Phosphoadenosine 5'-{3-[(3R)-3-hydroxy-4-({3-[(2-{[(3S)-3-hydroxybutanoyl]sulfanyl}ethyl)amino]-3-oxopropyl}amino)-2,2-dimethyl-4-oxobutyl] dihydrogen diphosphate}
- 3'-Phosphoadenosine 5'-{3-[(3R)-3-hydroxy-4-({3-[(2-{[(3S)-3-hydroxybutanoyl]sulfanyl}ethyl)amino]-3-oxopropyl}amino)-2,2-dimethyl-4-oxobutyl] dihydrogen diphosphoric acid}
- 3'-Phosphoadenosine 5'-{3-[(3R)-3-hydroxy-4-({3-[(2-{[(3S)-3-hydroxybutanoyl]sulphanyl}ethyl)amino]-3-oxopropyl}amino)-2,2-dimethyl-4-oxobutyl] dihydrogen diphosphate}
- 3'-Phosphoadenosine 5'-{3-[(3R)-3-hydroxy-4-({3-[(2-{[(3S)-3-hydroxybutanoyl]sulphanyl}ethyl)amino]-3-oxopropyl}amino)-2,2-dimethyl-4-oxobutyl] dihydrogen diphosphoric acid}
- 3-HYDROXYBUTANOYL-COENZYME A
- L(+)-3-Hydroxybutyroyl-CoA
- L(+)-b-Hydroxybutyroyl-CoA
- L(+)-beta-Hydroxybutyroyl-CoA
- L(+)-β-Hydroxybutyroyl-CoA
- L-3-Hydroxybutanoyl-CoA
|
|---|
|
Chemical Formula: |
C25H42N7O18P3S |
|---|
| Average Molecular Weight: |
853.623 |
|---|
| Monoisotopic Molecular
Weight: |
853.151987801 |
|---|
| InChI Key: |
QHHKKMYHDBRONY-VKBDFPRVSA-N |
|---|
| InChI: | InChI=1S/C25H42N7O18P3S/c1-13(33)8-16(35)54-7-6-27-15(34)4-5-28-23(38)20(37)25(2,3)10-47-53(44,45)50-52(42,43)46-9-14-19(49-51(39,40)41)18(36)24(48-14)32-12-31-17-21(26)29-11-30-22(17)32/h11-14,18-20,24,33,36-37H,4-10H2,1-3H3,(H,27,34)(H,28,38)(H,42,43)(H,44,45)(H2,26,29,30)(H2,39,40,41)/t13-,14+,18+,19+,20-,24+/m0/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (2R)-4-({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-2-hydroxy-N-{2-[(2-{[(3S)-3-hydroxybutanoyl]sulfanyl}ethyl)-C-hydroxycarbonimidoyl]ethyl}-3,3-dimethylbutanimidic acid |
|---|
|
Traditional IUPAC Name: |
(2R)-4-[({[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxy]-2-hydroxy-N-{2-[(2-{[(3S)-3-hydroxybutanoyl]sulfanyl}ethyl)-C-hydroxycarbonimidoyl]ethyl}-3,3-dimethylbutanimidic acid |
|---|
| SMILES: | [H][C@@](C)(O)CC(=O)SCCN=C(O)CCN=C(O)[C@]([H])(O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@@]1([H])O[C@@]([H])(N2C=NC3=C(N)N=CN=C23)[C@]([H])(O)[C@]1([H])OP(O)(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as acyl coas. These are organic compounds containing a coenzyme A substructure linked to an acyl chain. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
|
Direct Parent |
Acyl CoAs |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Coenzyme a or derivatives
- Purine ribonucleoside diphosphate
- Purine ribonucleoside 3',5'-bisphosphate
- N-glycosyl compound
- Glycosyl compound
- Organic pyrophosphate
- Monosaccharide phosphate
- 6-aminopurine
- Purine
- Imidazopyrimidine
- Monoalkyl phosphate
- Aminopyrimidine
- Imidolactam
- Alkyl phosphate
- Pyrimidine
- Primary aromatic amine
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic phosphate
- N-substituted imidazole
- Monosaccharide
- Saccharide
- Heteroaromatic compound
- Oxolane
- Imidazole
- Azole
- Thiocarboxylic acid ester
- Secondary alcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Sulfenyl compound
- Thioether
- Thiocarboxylic acid or derivatives
- Carboxylic acid derivative
- Carboximidic acid derivative
- Carboximidic acid
- Hydrocarbon derivative
- Primary amine
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Amine
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | 15453 | | HMDB ID | Not Available | | Pubchem Compound ID | 439419 | | Kegg ID | C01144 | | ChemSpider ID | 388532 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available | | Ligand Expo | 3HC |
|
|---|