|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB002040 |
|---|
|
Identification |
|---|
| Name: |
Aldehyde |
|---|
| Description: | A dialdehyde is an organic chemical compound with two aldehyde groups. The nomenclature of dialdehydes have the ending -dial or sometimes -dialdehyde. Short aliphatic dialdehydes are sometimes named after the diacid from which they can de derived. An example is butanedial, which is also called succinaldehyde (from succinic acid).; Aldehydes are readily identified by spectroscopic methods. Using IR spectroscopy, they display a strong ?CO band near 1700 cm??. In their 1H NMR spectra, the formyl hydrogen center absorbs near δ9, which is a distinctive part of the spectrum. This signal shows the characteristic coupling to any protons on the alpha carbon.; An aldehyde ( /??ld?ha?d/) is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center (a carbon double bonded to oxygen) bonded to hydrogen and an R group, which is any generic alkyl or side chain. The group without R is called the aldehyde group or formyl group. Aldehydes differ from ketones in that the carbonyl is placed at the end of a carbon skeleton rather than between two carbon atoms. Aldehydes are common in organic chemistry. Many fragrances are aldehydes. |
|---|
|
Structure |
|
|---|
| Synonyms: | - Aldehido
- Aldehidos
- Aldehyd
- Aldehyde
- Aldehydes
- Aldehydum
- An aldehyde
- Formaldehyde
- Formalin
- Methanal
- Methylene oxide
- Oxomethane
- Oxomethylene
- RC(2O)H
|
|---|
|
Chemical Formula: |
C20H30O5 |
|---|
| Average Molecular Weight: |
350.4492 |
|---|
| Monoisotopic Molecular
Weight: |
350.20932407 |
|---|
| InChI Key: |
LVLQYGYNBVIONY-PSPARDEHSA-N |
|---|
| InChI: | InChI=1S/C20H30O5/c21-17-10-6-2-1-3-7-12-18(22)13-8-4-5-9-14-19(23)15-11-16-20(24)25/h3-5,7-9,13-14,17-19,22-23H,1-2,6,10-12,15-16H2,(H,24,25)/b5-4+,7-3-,13-8+,14-9-/t18-,19-/m1/s1 |
|---|
| CAS
number: |
72379-22-7 |
|---|
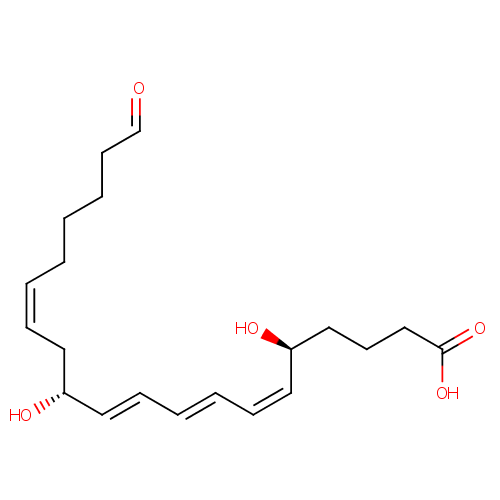
| IUPAC Name: | (5S,6Z,8E,10E,12R,14Z)-5,12-dihydroxy-20-oxoicosa-6,8,10,14-tetraenoic acid |
|---|
|
Traditional IUPAC Name: |
20-aldehyde leukotriene B4 |
|---|
| SMILES: | O[C@@H](CCCC(O)=O)\C=C/C=C/C=C/[C@H](O)C\C=C/CCCCC=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as leukotrienes. These are eicosanoids containing a hydroxyl group attached to the aliphatic chain of an arachidonic acid. Eicosanoids containing a hydroxyl group attached to the aliphatic chain of an arachidonic acid. Leukotrienes have four double bonds, three (and only three) of which are conjugated. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Fatty Acyls |
|---|
| Sub Class | Eicosanoids |
|---|
|
Direct Parent |
Leukotrienes |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Leukotriene
- Hydroxyeicosatetraenoic acid
- Long-chain fatty acid
- Hydroxy fatty acid
- Fatty acid
- Unsaturated fatty acid
- Alpha-hydrogen aldehyde
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aldehyde
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|