Search Results for compounds
Searching compounds for
returned 4373 results.
Displaying compounds 1001 - 1010 of
4373 in total
Ubiquinol-6 (PAMDB001855)
IUPAC:
2-[(2E,6E,10E,14E,18E)-3,7,11,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaen-1-yl]-5,6-dimethoxy-3-methylbenzene-1,4-diol
CAS: 5677-58-7
Description: Ubiquinol-6 is a member of the chemical class known as Polyprenylbenzoquinols. They are reduced forms of polyprenylbenzoquinines (ubiquinones). These are compounds containing a polyisoprene chain attached to a quinol at the second ring position. Ubiquiol-6 has just 6 isoprene units. Normally in Pseudomonas aeruginosa the active form of Ubiquinol has 8 isoprene units (Ubiquinol-8) and in humans it normally has 10. Ubiquinol-6 is a ??ailed??or incomplete version of Ubiquinol 8 that arises from conjugation by a shortened prenyl tail via 4-hydroxybenzoate polyprenyltransferase. Coenzyme Q(n) exists in three redox states, fully oxidized (ubiquinone), partially reduced (semiquinones or ubisemiquinones), and fully reduced (ubiquinols). The redox functions of ubiquinol in cellular energy production and antioxidant protection are based on the ability to exchange two electrons in a redox cycle between ubiquinol (reduced) and the ubiquinone (oxidized) form. Ubiquionols are important in cellular respiration. They are fat-soluble and therefore mobile in cellular membranes; they play a unique role in the electron transport chain (ETC). In the inner bacterial membrane, electrons from NADH and succinate pass through the ETC to the oxygen, which is then reduced to water. The transfer of electrons through ETC results in the pumping of H+ across the membrane creating a proton gradient across the membrane, which is used by ATP synthase (located on the membrane) to generate ATP.
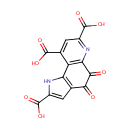
Pyrroloquinoline quinone (PAMDB001856)
IUPAC:
4,5-dioxo-1H,4H,5H-pyrrolo[2,3-f]quinoline-2,7,9-tricarboxylic acid
CAS: 72909-34-3
Description: Enzymes containing PQQ are called quinoproteins. PQQ and quinoproteins play a role in the redox metabolism and structural integrity of cells and tissues [PMID:2558842]. It was reported that aminoadipate semialdehyde dehydrogenase (AASDH) might also use PQQ as a cofactor, suggesting a possibility that PQQ is a vitamin in mammals. [PMID:12712191]
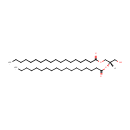
1,2-Dioctadecanoyl-sn-glycerol (PAMDB001861)
IUPAC:
(2S)-1-hydroxy-3-(octadecanoyloxy)propan-2-yl octadecanoate
CAS: Not Available
Description: 1,2-dioctadecanoyl-sn-glycerol is a member of the chemical class known as Diacylglycerols. These are glycerides consisting of two fatty acid chains covalently bonded to a glycerol molecule through ester linkages. 1,2-dioctadecanoyl-sn-glycerol is an intermediate in the synthesis of phopsholipids and glycerolipids with a pair of C18 chains.
1-(2-Carboxyphenylamino)-1'-deoxyribulose-5'-phosphate (PAMDB001862)
IUPAC:
2-{[3,4-dihydroxy-2-oxo-5-(phosphonatooxy)pentyl]amino}benzoate
CAS: Not Available
Description: 1-(2-carboxyphenylamino)-1'-deoxyribulose-5'-phosphate is a member of the chemical class known as Pentoses. These are monosaccharides in which the carbohydrate moiety contains five carbon atoms. It is an intermediate in the synthesis of tryptophan from chorismate.
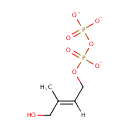
1-Hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate (PAMDB001868)
IUPAC:
{[(2E)-4-hydroxy-3-methylbut-2-en-1-yl phosphonato]oxy}phosphonate
CAS: Not Available
Description: 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate is a member of the chemical class known as Organic Pyrophosphates. These are organic compounds containing the pyrophosphate oxoanion, with the structure OP([O-])(=O)OP(O)([O-])=O. 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate is an intermediate in the mehtylerithritol phosphate pathway. . D-glyceraldehyde-3-phosphate and pyruvate are initially combined to yield 1-deoxy-D-xylylose 5-phosphate (DXP). DXP is then rearranged and reduced to generate the pathway's namesake compound, 2-C-methyl-D-erythritol 4-phosphate (MEP). In the third reaction MEP is converted into 4-diphosphocytidyl-2-C-methylerythritol, which is subsequently phosphorylated at the 2 position hydroxy group, yielding 4-diphosphocytidyl-2C-methylerythritol 2-phosphate. This product is then converted into 2-C-methyl-D-erythritol 2,4-cyclodiphosphate. This compound is then reduced to generate 1-hydroxy-2-methyl-2-(E)-butenyl 4-diphosphate. In the final step, this diphosphate compound is converted by a single enzyme into a 5-6:1 ratio of IPP and DMAPP This ratio is subsequently adjusted to 7:3 by isopentenyl diphosphate isomerase. Both IPP and DMAPP then become the basic building blocks of polyisoprenoid biosynthesis.
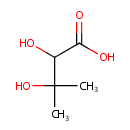
2,3-Dihydroxyisovaleric acid (PAMDB001875)
IUPAC:
2,3-dihydroxy-3-methylbutanoic acid
CAS: 1756-18-9
Description: 2,3-dihydroxyisovaleric acid (or 2,3-Dihydroxy-3-methylbutanoate) is invovled in branched chain amino acid biosynthesis. It is a substrate for Ketol-acid reductoisomerase (ilvC). This enzyme catalyzes the reaction: (R)-2,3-dihydroxy-3-methylbutanoate + NADP+ = (S)-2-hydroxy-2-methyl-3-oxobutanoate + NADPH.
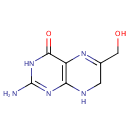
2-Amino-4-hydroxy-6-hydroxymethyl-7,8-dihydropteridine (PAMDB001880)
IUPAC:
2-amino-6-(hydroxymethyl)-3,4,7,8-tetrahydropteridin-4-one
CAS: 3672-03-5
Description: 2-amino-4-hydroxy-6-hydroxymethyl-7,8-dihydropteridine is a member of the chemical class known as Pterins and Derivatives. These are polycyclic aromatic compounds containing a pterin moeity, which consist of a pteridine ring bearing a ketone and an amine group to form 2-aminopteridin-4(3H)-one. 2-amino-4-hydroxy-6-hydroxymethyl-7,8-dihydropteridine is invovled in Folate biosynthesis. (KEGG)
3-Hydroxymyristic acid (PAMDB001888)
IUPAC:
3-hydroxytetradecanoic acid
CAS: 1961-72-4
Description: 3-hydroxymyristic acid is a member of the chemical class known as Hydroxy Fatty Acids. These are fatty acids in which the chain bears an hydroxyl group. Hydroxymyristic acid is a component of lipid A.
4-Amino-4-deoxy-L-arabinose (PAMDB001892)
IUPAC:
[({[(2R,3R,4S,5S)-5-amino-3,4-dihydroxyoxan-2-yl]oxy}(hydroxy)phosphoryl)oxy]({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(4-hydroxy-2-oxo-1,2-dihydropyrimidin-1-yl)oxolan-2-yl]methoxy})phosphinic acid
CAS: Not Available
Description: 4-amino-4-deoxy-L-arabinose is invovled in Modification of lipid A. Attachment of the cationic sugar 4-amino-4-deoxy-l-arabinose (l-Ara4N) to lipid A is required for the maintenance of polymyxin resistance in Pseudomonas aeruginosa. The enzyme called AmT synthesizes this molecule and transfers it to lipid A.
Margaric acid (PAMDB001901)
IUPAC:
heptadecanoic acid
CAS: 506-12-7
Description: Margaric acid, also known as heptadecanoic acid, is a saturated fatty acid with a 17-carbon chain. Exogenous propionate can be incorporated by Pseudomonas aeruginosa as a primer to produce lipids with fatty acids of odd chain lengths.