|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001856 |
|---|
|
Identification |
|---|
| Name: |
Pyrroloquinoline quinone |
|---|
| Description: | Enzymes containing PQQ are called quinoproteins. PQQ and quinoproteins play a role in the redox metabolism and structural integrity of cells and tissues [PMID:2558842]. It was reported that aminoadipate semialdehyde dehydrogenase (AASDH) might also use PQQ as a cofactor, suggesting a possibility that PQQ is a vitamin in mammals. [PMID:12712191] |
|---|
|
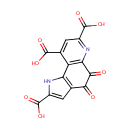
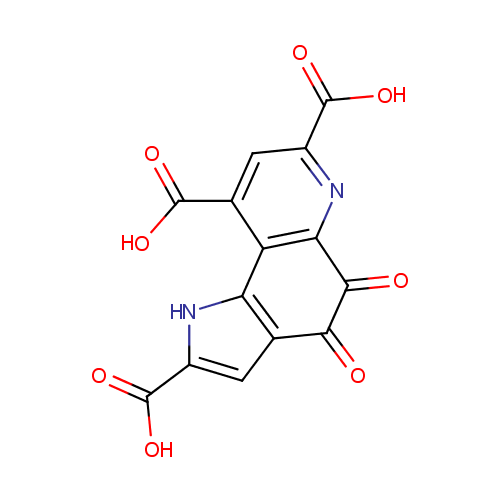
Structure |
|
|---|
| Synonyms: | - 2,7,9-Tricarboxy-1H-pyrrolo[2,3-f]quinoline-4,5-dione
- Methoxatin
- PQQ
- Pyrrolo-quinoline quinone
- Pyrroloquinoline-quinone
|
|---|
|
Chemical Formula: |
C14H6N2O8 |
|---|
| Average Molecular Weight: |
330.206 |
|---|
| Monoisotopic Molecular
Weight: |
330.012415178 |
|---|
| InChI Key: |
MMXZSJMASHPLLR-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C14H6N2O8/c17-10-4-2-6(14(23)24)15-8(4)7-3(12(19)20)1-5(13(21)22)16-9(7)11(10)18/h1-2,15H,(H,19,20)(H,21,22)(H,23,24) |
|---|
| CAS
number: |
72909-34-3 |
|---|
| IUPAC Name: | 4,5-dioxo-1H,4H,5H-pyrrolo[2,3-f]quinoline-2,7,9-tricarboxylic acid |
|---|
|
Traditional IUPAC Name: |
pyrroloquinoline quinone |
|---|
| SMILES: | OC(=O)C1=CC2=C(N1)C1=C(N=C(C=C1C(O)=O)C(O)=O)C(=O)C2=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as pyrroloquinoline quinones. These are compounds with a structure based on the 2,7,-tricarboxy-1H-pyrrolo[2,3-f ]quinoline-4,5-dione. Pyrroloquinoline Quinones usually bear a carboxylic acid group at the C-2, C-7 and C-9 positions. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Quinolines and derivatives |
|---|
| Sub Class | Pyrroloquinolines |
|---|
|
Direct Parent |
Pyrroloquinoline quinones |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Pyrroloquinoline quinone
- Quinoline-4-carboxylic acid
- Quinoline-2-carboxylic acid
- Tricarboxylic acid or derivatives
- Pyridine carboxylic acid or derivatives
- Pyridine carboxylic acid
- Indole or derivatives
- Aryl ketone
- Quinone
- Pyrrole-2-carboxylic acid or derivatives
- Pyrrole-2-carboxylic acid
- O-quinone
- Substituted pyrrole
- Pyridine
- Heteroaromatic compound
- Vinylogous amide
- Pyrrole
- Ketone
- Azacycle
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -3 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kasahara, T., Kato, T. (2003). "Nutritional biochemistry: A new redox-cofactor vitamin for mammals." Nature 422:832. Pubmed: 12712191
- Paz, M. A., Fluckiger, R., Torrelio, B. M., Gallop, P. M. (1989). "Methoxatin (PQQ), coenzyme for copper-dependent amine and mixed-function oxidation in mammalian tissues." Connect Tissue Res 20:251-257. Pubmed: 2558842
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|