Nitrite (PAMDB000465)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000465 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||
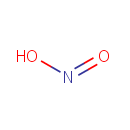
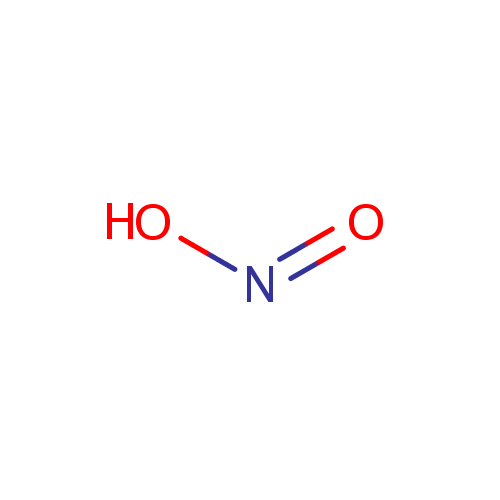
| Name: | Nitrite | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | A nitrite compound is either a salt or an ester of nitrous acid. Sodium nitrite is used for the curing of meat because it prevents bacterial growth and, in a reaction with the meat's myoglobin, gives the product a desirable dark red color. Nitrite can be reduced to nitric oxide or ammonia by many species of bacteria. Several mechanisms for nitrite conversion to NO have been described including enzymatic reduction by xanthine oxidoreductase and NO synthase (NOS), as well as nonenzymatic acidic disproportionation. -- Wikipedia | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | NO2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 46.0055 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 45.992903249 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | IOVCWXUNBOPUCH-UHFFFAOYSA-M | |||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/HNO2/c2-1-3/h(H,2,3)/p-1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 14797-65-0 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | nitrous acid | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | nitrous acid | |||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | [O-]N=O | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of inorganic compounds known as homogeneous other non-metal compounds. These are inorganic non-metallic compounds in which the largest atom belongs to the class of 'other nonmetals'. | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Inorganic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Homogeneous non-metal compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Homogeneous other non-metal compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Homogeneous other non-metal compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Acyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 2 Hydrogen ion + Nitrate + Ubiquinol-8 > Water + Nitrite + Ubiquinone-8 +2 Hydrogen ion 2 Hydrogen ion + Menaquinol 8 + Nitrate > Water + Menaquinone 8 + Nitrite +2 Hydrogen ion Ubiquinol-8 + Nitrate > Ubiquinone-8 + Water + Nitrite Menaquinol 8 + Nitrate > Menaquinone 8 + Water + Nitrite 5 Hydrogen ion + 3 NADH + Nitrite >2 Water +3 NAD + Ammonium 3 Ubiquinol-8 + 2 Hydrogen ion + Nitrite >3 Ubiquinone-8 +2 Water + Ammonium 3 Menaquinol 8 + 2 Hydrogen ion + Nitrite >3 Menaquinone 8 +2 Water + Ammonium Nitrite + Acceptor + Water + Acceptor <> Nitrate + Reduced acceptor + Reduced acceptor 2 Ferricytochrome c + Nitrite + Water <> Nitrate +2 Ferrocytochrome c +2 Hydrogen ion 4-Nitrocatechol + Oxygen + 3 Hydrogen ion <> Benzene-1,2,4-triol + Nitrite + Water Ammonia + 2 Water + 6 Ferricytochrome c + Ferricytochrome c <> Nitrite +6 Ferrocytochrome c +6 Hydrogen ion + Ferrocytochrome c Nitrobenzene + Oxygen <> Pyrocatechol + Nitrite Nitrite + Water + Cytochromes-C-Oxidized <> Nitrate + Cytochromes-C-Reduced a menaquinol + Nitrate + Hydrogen ion > a menaquinone + Nitrite + Water + Hydrogen ion Nitrate + a ubiquinol > Nitrite + Water + a ubiquinone Nitrate + Hydrogen ion > Nitrite + Water NAD(P)H + Nitrite + Hydrogen ion > NAD(P)<sup>+</sup> + Ammonium + Water Nitrite + acceptor > Nitrate + reduced acceptor Ammonia + 2 Water + 6 Ferricytochrome c > Nitrite +6 Ferrocytochrome c +7 Hydrogen ion More...Ammonia + NAD + 3 NADP + 2 Water <> Nitrite + NADH +3 NADPH +5 Hydrogen ion Nitrite + Nitrite > Nitrite Nitrate + cytochrome c nitrite reductase + Nitrate <> Nitrite + cytochrome c nitrite reductase + Water + Nitrite Nitrite + 3 NADH + 5 Hydrogen ion + Nitrite Ammonia +2 Water +3 NAD Nitrite + 6 ferrocytochrome c + 7 Hydrogen ion + Nitrite + 6 Ferrocytochrome c <> Ammonia +6 ferricytochrome c +2 Water +6 Ferricytochrome c | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Nakata, Sumio; Eiki, Toshio; Tanaka, Norihiro; Koyama, Tadashi; Tsukui, Hiroto; Watanabe, Shizuo. Production of nitrite ions from trinitrophenyl myosin and from trinitrophenyl subfragment-1. Journal of Biochemistry (Tokyo, Japan) (1986), 99(1), 27- | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in oxidation-reduction process
- Specific function:
- Ammonium hydroxide + 3 NAD(P)(+) + H(2)O = nitrite + 3 NAD(P)H
- Gene Name:
- nirB
- Locus Tag:
- PA1781
- Molecular weight:
- 89.7 kDa
Reactions
| Ammonium hydroxide + 3 NAD(P)(+) + H(2)O = nitrite + 3 NAD(P)H. |
- General function:
- Involved in oxidoreductase activity
- Specific function:
- The nitrate reductase enzyme complex allows Pseudomonas aeruginosa to use nitrate as an electron acceptor during anaerobic growth. The alpha chain is the actual site of nitrate reduction
- Gene Name:
- narG
- Locus Tag:
- PA3875
- Molecular weight:
- 141 kDa
Reactions
| Nitrite + acceptor = nitrate + reduced acceptor. |
- General function:
- Involved in nitrite reductase [NAD(P)H] activity
- Specific function:
- Required for activity of the reductase
- Gene Name:
- nirD
- Locus Tag:
- PA1780
- Molecular weight:
- 11.6 kDa
Reactions
| Ammonium hydroxide + 3 NAD(P)(+) + H(2)O = nitrite + 3 NAD(P)H. |
- General function:
- Involved in iron-sulfur cluster binding
- Specific function:
- The nitrate reductase enzyme complex allows Pseudomonas aeruginosa to use nitrate as an electron acceptor during anaerobic growth. The beta chain is an electron transfer unit containing four cysteine clusters involved in the formation of iron-sulfur centers. Electrons are transferred from the gamma chain to the molybdenum cofactor of the alpha subunit
- Gene Name:
- narH
- Locus Tag:
- PA3874
- Molecular weight:
- 58.1 kDa
Reactions
| Nitrite + acceptor = nitrate + reduced acceptor. |
- General function:
- Involved in nitrate reductase activity
- Specific function:
- The nitrate reductase enzyme complex allows Pseudomonas aeruginosa to use nitrate as an electron acceptor during anaerobic growth. The gamma chain is a membrane-embedded heme-iron unit resembling cytochrome b, which transfers electrons from quinones to the beta subunit
- Gene Name:
- narI
- Locus Tag:
- PA3872
- Molecular weight:
- 25 kDa
Reactions
| Nitrite + acceptor = nitrate + reduced acceptor. |
- General function:
- Involved in oxidoreductase activity
- Specific function:
- Catalytic subunit of the periplasmic nitrate reductase (NAP). Only expressed at high levels during aerobic growth. NapAB complex receives electrons from the membrane-anchored tetraheme protein napC, thus allowing electron flow between membrane and periplasm. Essential function for nitrate assimilation and may have a role in anaerobic metabolism
- Gene Name:
- napA
- Locus Tag:
- PA1174
- Molecular weight:
- 92.9 kDa
Reactions
| Nitrite + acceptor = nitrate + reduced acceptor. |
- General function:
- Energy production and conversion
- Specific function:
- Small subunit of the periplasmic nitrate reductase (NAP). Only expressed at high levels during aerobic growth. NapAB complex receives electrons from the membrane-anchored tetraheme napC protein, thus allowing electron flow between membrane and periplasm. Essential function for nitrate assimilation and may have a role in anaerobic metabolism
- Gene Name:
- napB
- Locus Tag:
- PA1173
- Molecular weight:
- 17.9 kDa
- General function:
- Involved in heme binding
- Specific function:
- Mediates electron flow from quinones to the napAB complex
- Gene Name:
- napC
- Locus Tag:
- PA1172
- Molecular weight:
- 22.7 kDa
- General function:
- Involved in unfolded protein binding
- Specific function:
- Chaperone required for proper molybdenum cofactor insertion and final assembly of the membrane-bound respiratory nitrate reductase 1. Required for the insertion of the molybdenum into the apo-NarG subunit, maybe by keeping NarG in an appropriate competent-open conformation for the molybdenum cofactor insertion to occur. NarJ maintains the apoNarGH complex in a soluble state. Upon insertion of the molybdenum cofactor, NarJ seems to dissociate from the activated soluble NarGH complex, before its association with the NarI subunit on the membrane
- Gene Name:
- narJ
- Locus Tag:
- PA3873
- Molecular weight:
- 27.3 kDa
Transporters
- General function:
- Involved in transporter activity
- Specific function:
- May act as a nitrite transporter
- Gene Name:
- nirC
- Locus Tag:
- PA0517
- Molecular weight:
- 12.8 kDa
- General function:
- Involved in transmembrane transport
- Specific function:
- Involved in excretion of nitrite produced by the dissimilatory reduction of nitrate
- Gene Name:
- narK
- Locus Tag:
- PA3876
- Molecular weight:
- 50.6 kDa