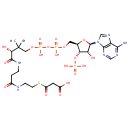
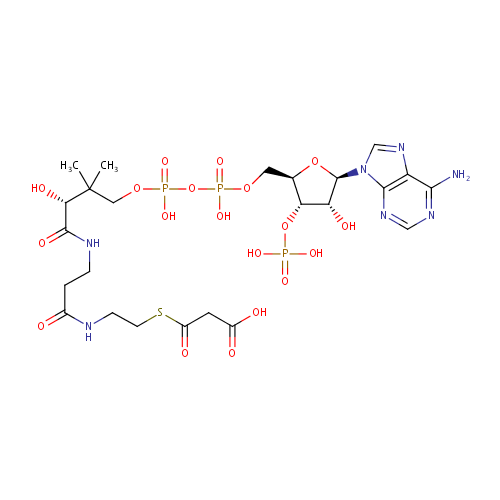
Malonyl-CoA (PAMDB000284)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000284 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Malonyl-CoA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Malonyl-CoA is a coenzyme A derivative which plays a key role in fatty acid synthesis in Pseudomonas aeruginosa Fatty acids must be activated with CoA before any chemical modification can be applied. Also fatty acid metabolic intermediates will also exists as CoA derivatives until the CoA is enzymatically cleaved. The fatty acid group is linked to the terminal thiol moiety of CoA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C24H38N7O19P3S | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 853.58 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 853.115602295 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | LTYOQGRJFJAKNA-VFLPNFFSSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C24H38N7O19P3S/c1-24(2,19(37)22(38)27-4-3-13(32)26-5-6-54-15(35)7-14(33)34)9-47-53(44,45)50-52(42,43)46-8-12-18(49-51(39,40)41)17(36)23(48-12)31-11-30-16-20(25)28-10-29-21(16)31/h10-12,17-19,23,36-37H,3-9H2,1-2H3,(H,26,32)(H,27,38)(H,33,34)(H,42,43)(H,44,45)(H2,25,28,29)(H2,39,40,41)/t12-,17-,18-,19?,23-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 524-14-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 3-[(2-{3-[(2R)-3-[({[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)methyl]-2-hydroxy-3-methylbutanamido]propanamido}ethyl)sulfanyl]-3-oxopropanoic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | malonyl-coa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC(C)(COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N)C(O)C(=O)NCCC(=O)NCCSC(=O)CC(O)=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as acyl coas. These are organic compounds containing a coenzyme A substructure linked to an acyl chain. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Fatty Acyls | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Fatty acyl thioesters | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Acyl CoAs | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Acetyl-CoA + Adenosine triphosphate + Hydrogen carbonate <> ADP + Hydrogen ion + Malonyl-CoA + Phosphate acyl carrier protein + Malonyl-CoA <> Coenzyme A + Malonyl-[acyl-carrier protein] S-Adenosylmethionine + Malonyl-CoA > S-Adenosylhomocysteine + malonyl-CoA methyl ester Adenosine triphosphate + Acetyl-CoA + Hydrogen carbonate <> ADP + Phosphate + Malonyl-CoA Malonyl-CoA + Acyl-carrier protein + Acyl-carrier protein <> Coenzyme A + Malonyl-[acyl-carrier protein] + Malonyl-[acyl-carrier protein] Acetyl-CoA + Carboxybiotin-carboxyl-carrier protein <> Malonyl-CoA + Holo-[carboxylase] Adenosine triphosphate + Acetyl-CoA + Carbonic acid > ADP + Inorganic phosphate + Malonyl-CoA Acetyl-CoA + Hydrogen carbonate + Adenosine triphosphate > Adenosine diphosphate + Phosphate + Hydrogen ion + Malonyl-CoA + ADP + Malonyl-CoA Malonyl-CoA + a holo-[acyl-carrier protein]? + Malonyl-CoA > Coenzyme A + a malonyl-[acp] Malonyl-CoA + a holo-[acyl-carrier protein] + Malonyl-CoA > Coenzyme A + a malonyl-[acp] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Hulsmann, W. C. Synthesis of malonyl coenzyme A from acetyl coenzyme A and oxalosuccinate in mitochondria. Biochimica et Biophysica Acta (1963), 77(3), 502-3. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in acetyl-CoA carboxylase activity
- Specific function:
- Controls translation of mRNA for both itself and the alpha-subunit (accA) by binding to a probable hairpin in the 5' of the mRNA. Binding to mRNA inhibits translation; this is partially relieved by acetyl-CoA. Increasing amounts of mRNA also inhibit enzyme activity
- Gene Name:
- accD
- Locus Tag:
- PA3112
- Molecular weight:
- 31.8 kDa
Reactions
| ATP + acetyl-CoA + HCO(3)(-) = ADP + phosphate + malonyl-CoA. |
- General function:
- Involved in transferase activity
- Specific function:
- Malonyl-CoA + [acyl-carrier-protein] = CoA + malonyl-[acyl-carrier-protein]
- Gene Name:
- fabD
- Locus Tag:
- PA2968
- Molecular weight:
- 32.4 kDa
Reactions
| Malonyl-CoA + [acyl-carrier-protein] = CoA + malonyl-[acyl-carrier-protein]. |
- General function:
- Involved in acetyl-CoA carboxylase activity
- Specific function:
- Component of the acetyl coenzyme A carboxylase (ACC) complex. First, biotin carboxylase catalyzes the carboxylation of biotin on its carrier protein (BCCP) and then the CO(2) group is transferred by the carboxyltransferase to acetyl-CoA to form malonyl-CoA
- Gene Name:
- accA
- Locus Tag:
- PA3639
- Molecular weight:
- 34.9 kDa
Reactions
| ATP + acetyl-CoA + HCO(3)(-) = ADP + phosphate + malonyl-CoA. |
- General function:
- Involved in acetyl-CoA carboxylase activity
- Specific function:
- This protein is a component of the acetyl coenzyme A carboxylase complex; first, biotin carboxylase catalyzes the carboxylation of the carrier protein and then the transcarboxylase transfers the carboxyl group to form malonyl-CoA
- Gene Name:
- accB
- Locus Tag:
- PA4847
- Molecular weight:
- 16.5 kDa
- General function:
- Involved in ligase activity
- Specific function:
- This protein is a component of the acetyl coenzyme A carboxylase complex; first, biotin carboxylase catalyzes the carboxylation of the carrier protein and then the transcarboxylase transfers the carboxyl group to form malonyl-CoA
- Gene Name:
- accC
- Locus Tag:
- PA4848
- Molecular weight:
- 48.9 kDa
Reactions
| ATP + biotin-[carboxyl-carrier-protein] + CO(2) = ADP + phosphate + carboxy-biotin-[carboxyl-carrier-protein]. |
| ATP + acetyl-CoA + HCO(3)(-) = ADP + phosphate + malonyl-CoA. |
- General function:
- Involved in fatty acid biosynthetic process
- Specific function:
- Carrier of the growing fatty acid chain in fatty acid biosynthesis
- Gene Name:
- acpP
- Locus Tag:
- PA2966
- Molecular weight:
- 8.7 kDa