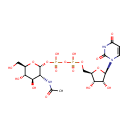
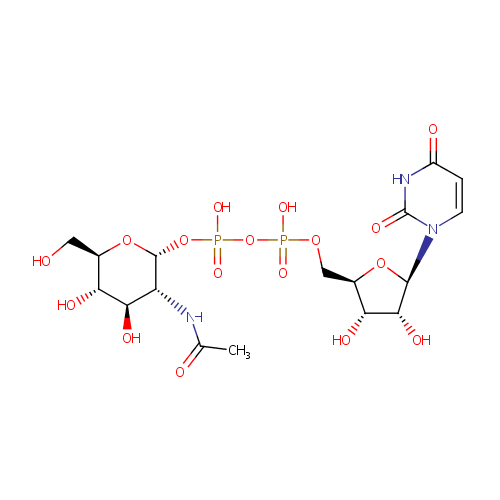
Uridine diphosphate-N-acetylglucosamine (PAMDB000120)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000120 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Uridine diphosphate-N-acetylglucosamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Uridine diphosphate N-acetylglucosamine or UDP-GlcNAc is a nucleotide sugar and a coenzyme in metabolism. It is used by glycosyltransferases to transfer N-acetylglucosamine residues to substrates. D-Glucosamine is made naturally in the form of glucosamine-6-phosphate, and is the biochemical precursor of all nitrogen-containing sugars. Specifically, glucosamine-6-phosphate is synthesized from fructose 6-phosphate and glutamine as the first step of the hexosamine biosynthesis pathway. The end-product of this pathway is UDP-GlcNAc, which is then used for making glycosaminoglycans, proteoglycans, and glycolipids. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C17H27N3O17P2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 607.3537 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 607.081569477 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | LFTYTUAZOPRMMI-CFRASDGPSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C17H27N3O17P2/c1-6(22)18-10-13(26)11(24)7(4-21)35-16(10)36-39(31,32)37-38(29,30)33-5-8-12(25)14(27)15(34-8)20-3-2-9(23)19-17(20)28/h2-3,7-8,10-16,21,24-27H,4-5H2,1H3,(H,18,22)(H,29,30)(H,31,32)(H,19,23,28)/t7-,8-,10-,11-,12-,13-,14-,15-,16-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 528-04-1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | [({[(2R,3S,4R,5R)-5-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-1-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]({[(2R,3R,4R,5S,6R)-3-acetamido-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy})phosphinic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | udp-N-acetyl-α-D-glucosamine | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC(=O)N[C@@H]1[C@@H](O)[C@H](O)[C@@H](CO)O[C@@H]1OP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1O)N1C=CC(=O)NC1=O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as pyrimidine nucleotide sugars. These are pyrimidine nucleotides bound to a saccharide derivative through the terminal phosphate group. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Nucleosides, nucleotides, and analogues | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Pyrimidine nucleotides | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Pyrimidine nucleotide sugars | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Pyrimidine nucleotide sugars | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | (R)-3-Hydroxytetradecanoyl-[acyl-carrier protein] + Uridine diphosphate-N-acetylglucosamine <> acyl carrier protein + UDP-3-O-(3-Hydroxymyristoyl)-N-acetylglucosamine Uridine diphosphate-N-acetylglucosamine + Undecaprenyl-diphospho-N-acetylmuramoyl-L-alanyl-D-glutamyl-meso-2,6-diaminopimeloyl-D-alanyl-D-alanine > Hydrogen ion + Undecaprenyl-diphospho-N-acetylmuramoyl-(N-acetylglucosamine)-L-alanyl-D-glutamyl-meso-2,6-diaminopimeloyl-D-alanyl-D-alanine + Uridine 5'-diphosphate Water + Uridine diphosphate-N-acetylglucosamine > N-Acetyl-glucosamine 1-phosphate +2 Hydrogen ion + Uridine 5'-monophosphate Phosphoenolpyruvic acid + Uridine diphosphate-N-acetylglucosamine <> Phosphate + UDP-N-Acetyl-3-(1-carboxyvinyl)-D-glucosamine N-Acetyl-glucosamine 1-phosphate + Hydrogen ion + Uridine triphosphate > Pyrophosphate + Uridine diphosphate-N-acetylglucosamine Uridine diphosphate-N-acetylglucosamine + Undecaprenyl phosphate > Uridine 5'-monophosphate + Undecaprenyl-N-acetyl-alpha-D-glucosaminyl-pyrophosphate Uridine diphosphate-N-acetylglucosamine <> UDP-N-Acetyl-D-mannosamine Uridine diphosphate-N-acetylglucosamine + Water <> N-Acetylmannosamine + Uridine 5'-diphosphate Uridine triphosphate + Glucosamine-1P <> Pyrophosphate + Uridine diphosphate-N-acetylglucosamine (3R)-3-Hydroxytetradecanoyl-[acyl-carrier protein] + Uridine diphosphate-N-acetylglucosamine + (3R)-3-Hydroxytetradecanoyl-[acyl-carrier protein] <> Acyl-carrier protein + UDP-3-O-(3-Hydroxymyristoyl)-N-acetylglucosamine + Acyl-carrier protein Undecaprenyl-diphospho-N-acetylmuramoyl-L-alanyl-D-glutamyl-meso-2,6-diaminopimeloyl-D-alanyl-D-alanine + Uridine diphosphate-N-acetylglucosamine <> Undecaprenyl-diphospho-N-acetylmuramoyl-(N-acetylglucosamine)-L-alanyl-D-glutamyl-meso-2,6-diaminopimeloyl-D-alanyl-D-alanine + Uridine 5'-diphosphate MurAc(oyl-L-Ala-D-gamma-Glu-L-Lys-D-Ala-D-Ala)-diphospho-undecaprenol + Uridine diphosphate-N-acetylglucosamine <> Undecaprenyl-diphospho-N-acetylmuramoyl-(N-acetylglucosamine)-L-alanyl-gamma-D-glutamyl-L-lysyl-D-alanyl-D-alanine + Uridine 5'-diphosphate Uridine diphosphate-N-acetylglucosamine + Di-trans,poly-cis-undecaprenyl phosphate <> N-Acetyl-D-glucosaminyldiphosphoundecaprenol + Uridine 5'-monophosphate + N-Acetyl-D-glucosaminyldiphospho-di-trans,octa-cis-undecaprenol Uridine diphosphate-N-acetylglucosamine poly-β-1,6-N-acetyl-D-glucosamine + Uridine 5'-diphosphate a lipopolysaccharide + Uridine diphosphate-N-acetylglucosamine a <i>N</i>-acetyl-D-glucosaminyl-lipopolysaccharide + Uridine 5'-diphosphate Di-trans,poly-cis-undecaprenyl phosphate + Uridine diphosphate-N-acetylglucosamine <> Undecaprenyl-N-acetyl-alpha-D-glucosaminyl-pyrophosphate + Uridine 5'-monophosphate N-Acetylmuramoyl-L-alanyl-D-glutamyl-meso-2,6-diaminopimelyl-D-alanyl-D-alanine-diphosphoundecaprenol + Uridine diphosphate-N-acetylglucosamine <> Hydrogen ion + N-Acetylmuramoyl-L-alanyl-D-glutamyl-L-lysyl-D-alanyl-D-alanine-diphosphoundecaprenyl-N-acetylglucosamine + Uridine 5'-diphosphate Hydrogen ion + <i>N</i>-acetyl-α-D-glucosamine 1-phosphate + Uridine triphosphate > Uridine diphosphate-N-acetylglucosamine + Pyrophosphate (R)-3-hydroxytetradecanoyl-[acyl-carrier-protein] + Uridine diphosphate-N-acetylglucosamine > [acyl-carrier-protein] + UDP-3-O-(3-Hydroxymyristoyl)-N-acetylglucosamine Phosphoenolpyruvic acid + Uridine diphosphate-N-acetylglucosamine > Inorganic phosphate + UDP-N-Acetyl-3-(1-carboxyvinyl)-D-glucosamine Uridine diphosphate-N-acetylglucosamine + Mur2Ac(oyl-L-Ala-gamma-D-Glu-L-Lys-D-Ala-D-Ala)-diphosphoundecaprenol > Uridine 5'-diphosphate + GlcNAc-(1->4)-Mur2Ac(oyl-L-Ala-gamma-D-Glu-L-Lys-D-Ala-D-Ala)-diphosphoundecaprenol More...Uridine diphosphate-N-acetylglucosamine + LPS (1-O-antigen) > Uridine 5'-diphosphate + N-acetyl-D-glucosaminyllipopolysaccharide Uridine diphosphate-N-acetylglucosamine + Mur2Ac(oyl-L-Ala-gamma-D-Glu-L-Lys-D-Ala-D-Ala)-diphosphoundecaprenol <> Uridine 5'-diphosphate + Undecaprenyl-diphospho-N-acetylmuramoyl-(N-acetylglucosamine)-L-alanyl-gamma-D-glutamyl-L-lysyl-D-alanyl-D-alanine Uridine diphosphate-N-acetylglucosamine + LPS (1-O-antigen) <> Uridine 5'-diphosphate Uridine diphosphate-N-acetylglucosamine + (3R)-3-hydroxymyristoyl-[acp] > a holo-[acyl-carrier protein] + UDP-3-O-[(3R)-3-hydroxymyristoyl]-N-acetyl-α-D-glucosamine N-Acetyl-glucosamine 1-phosphate + Uridine triphosphate + Hydrogen ion + N-Acetyl-glucosamine 1-phosphate + Uridine triphosphate > Uridine diphosphate-N-acetylglucosamine + Pyrophosphate Uridine diphosphate-N-acetylglucosamine <> UDP-N-acetyl-D-mannosamine + UDP-N-Acetyl-D-mannosamine Uridine diphosphate-N-acetylglucosamine + Phosphoenolpyruvic acid > Phosphate + UDP-N-acetyl-α-D-glucosamine-enolpyruvate Undecaprenyl-diphospho-N-acetylmuramoyl-L-alanyl-D-glutamyl-meso-2,6-diaminopimeloyl-D-alanyl-D-alanine + Uridine diphosphate-N-acetylglucosamine + Undecaprenyl-diphospho-N-acetylmuramoyl-L-alanyl-D-glutamyl-meso-2,6-diaminopimeloyl-D-alanyl-D-alanine > Uridine 5'-diphosphate + Hydrogen ion + lipid II(A) + Uridine 5'-diphosphate di-trans,octa-cis-undecaprenyl phosphate + Uridine diphosphate-N-acetylglucosamine > Uridine 5'-monophosphate + N-acetyl-α-D-glucosaminyl-diphospho-ditrans,octacis-undecaprenol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Takenouchi, Kenji; Ishige, Kazuya; Midorikawa, Yuichiro; Okuyama, Kiyoshi; Hamamoto, Tomoki; Noguchi, Toshitada. Process for producing uridine diphosphate-N-acetylglucosamine. PCT Int. Appl. (1999), 38 pp. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in transferase activity
- Specific function:
- Involved in the biosynthesis of lipid A, a phosphorylated glycolipid that anchors the lipopolysaccharide to the outer membrane of the cell
- Gene Name:
- lpxA
- Locus Tag:
- PA3644
- Molecular weight:
- 28 kDa
Reactions
| (R)-3-hydroxytetradecanoyl-[acyl-carrier-protein] + UDP-N-acetylglucosamine = [acyl-carrier-protein] + UDP-3-O-(3-hydroxytetradecanoyl)-N-acetylglucosamine. |
- General function:
- Involved in transferase activity, transferring alkyl or aryl (other than methyl) groups
- Specific function:
- Cell wall formation. Adds enolpyruvyl to UDP-N- acetylglucosamine. Target for the antibiotic phosphomycin
- Gene Name:
- murA
- Locus Tag:
- PA4450
- Molecular weight:
- 44.6 kDa
Reactions
| Phosphoenolpyruvate + UDP-N-acetyl-D-glucosamine = phosphate + UDP-N-acetyl-3-O-(1-carboxyvinyl)-D-glucosamine. |
- General function:
- Involved in magnesium ion binding
- Specific function:
- Catalyzes the last two sequential reactions in the de novo biosynthetic pathway for UDP-GlcNAc. Responsible for the acetylation of Glc-N-1-P to give GlcNAc-1-P and for the uridyl transfer from UTP to GlcNAc-1-P which produces UDP-GlcNAc
- Gene Name:
- glmU
- Locus Tag:
- PA5552
- Molecular weight:
- 48.9 kDa
Reactions
| Acetyl-CoA + alpha-D-glucosamine 1-phosphate = CoA + N-acetyl-alpha-D-glucosamine 1-phosphate. |
| UTP + N-acetyl-alpha-D-glucosamine 1-phosphate = diphosphate + UDP-N-acetyl-D-glucosamine. |
- General function:
- Involved in undecaprenyldiphospho-muramoylpentapeptide beta-N-acetylglucosaminyltransferase activity
- Specific function:
- Cell wall formation. Catalyzes the transfer of a GlcNAc subunit on undecaprenyl-pyrophosphoryl-MurNAc-pentapeptide (lipid intermediate I) to form undecaprenyl-pyrophosphoryl-MurNAc- (pentapeptide)GlcNAc (lipid intermediate II)
- Gene Name:
- murG
- Locus Tag:
- PA4412
- Molecular weight:
- 37.8 kDa
Reactions
| UDP-N-acetylglucosamine + Mur2Ac(oyl-L-Ala-gamma-D-Glu-L-Lys-D-Ala-D-Ala)-diphosphoundecaprenol = UDP + GlcNAc-(1->4)-Mur2Ac(oyl-L-Ala-gamma-D-Glu-L-Lys-D-Ala-D-Ala)-diphosphoundecaprenol. |
- General function:
- Involved in fatty acid biosynthetic process
- Specific function:
- Carrier of the growing fatty acid chain in fatty acid biosynthesis
- Gene Name:
- acpP
- Locus Tag:
- PA2966
- Molecular weight:
- 8.7 kDa