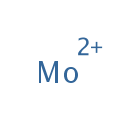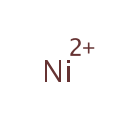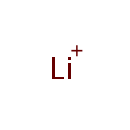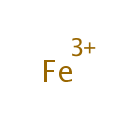Search Results for compounds
Searching compounds for
returned 4373 results.
Displaying compounds 981 - 990 of
4373 in total
Molybdenum (PAMDB001785)
IUPAC:
molybdenum(2+) ion
CAS: 7439-98-7
Description: Molybdenum is a transition metal with the atomic symbol Mo, atomic number 42, and atomic weight 95.94. The pure metal is silvery white in color, fairly soft, and has one of the highest melting points of all pure elements. Physiologically, it exists as an ion. It is an essential trace element, being a component of the enzymes xanthine oxidase, aldehyde oxidase, and nitrate reductase. There is a trace requirement for molybdenum in plants, and soils can be barren due to molybdenum deficiencies. Plants and animals generally have molybdenum present in amounts of a few parts per million. In small quantities, molybdenum is effective at hardening steel. Molybdenum is important in plant nutrition, and is found in certain enzymes, including xanthine oxidase. Molybdenum is used to this day in high-strength alloys and in high-temperature steels. Special molybdenum-containing alloys, such as the Hastelloys, are notably heat-resistant and corrosion-resistant. Molybdenum is used in oil pipelines, aircraft and missile parts, and in filaments. Molybdenum finds use as a catalyst in the petroleum industry, especially in catalysts for removing organic sulfurs from petroleum products. It is used to form the anode in some x-ray tubes, particularly in mammography applications. And is found in some electronic applications as the conductive metal layers in thin-film transistors (TFTs). Molybdenum disulfide is a good lubricant, especially at high temperatures. And Mo-99 is used in the nuclear isotope industry. Molybdenum pigments range from red-yellow to a bright red orange and are used in paints, inks, plastics, and rubber compounds.
Nickel (PAMDB001786)
IUPAC:
nickel(2+) ion
CAS: 7440-02-0
Description: Nickel is a solid, silver-white, hard, malleable transition metal with an atomic number of 28. It resists corrosion even at high temperatures. It is present in many alloys in widespread use, including stainless steels. It may also be present as an impurity in any alloy. Nickel is used in the production of coins, jewellery, and nickel-cadmium batteries, and as a catalyst for the hydrogenation of liquid oils to solid fats such as oleomargarine and vegetable shortening. Nickel-containing dental alloys continue to be used successfully in the provision of various forms of dental care. Many of these alloys have applications in the construction of restorations designed to remain in clinical service for many years, including crowns, fixed bridgework, and removable partial dentures. Furthermore, nickel containing alloys find extensive application in orthodontics, including metallic brackets, arch wires, bands, springs and ligature wires. Many instruments and devises, for example, endodontic instruments also contain nickel.
Lithium (PAMDB001788)
IUPAC:
lithium(1+) ion
CAS: 7439-93-2
Description: Lithium (Li) is an alkali metal with atomic number 3. Trace amounts of lithium are present in all organisms, though the element serves no apparent vital biological function. (Wikipedia) Scientific research has suggested that lithium is an inhibitor of several Pseudomonas aeruginosa enzymes, such as L-rhamnulose-1-phosphate aldolase. (EcoCyc)
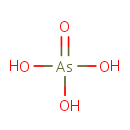
Arsenate (PAMDB001789)
IUPAC:
arsoric acid
CAS: 15584-04-0
Description: Arsenate is a member of the chemical class known as Miscellaneous Arsenites. These are inorganic compounds in which the largest metallic oxoanion is arsenite, to which either no atom or a non metal atom is bonded. The arsenate ion is AsO43-. An arsenate (compound) is any compound that contains this ion. Arsenates are salts or esters of arsenic acid. The arsenic atom in arsenate has a valency of 5 and is also known as pentavalent arsenic or As[V]. Arsenate resembles phosphate in many respects, since arsenic and phosphorus occur in the same group (column) of the periodic table. Arsenates are moderate oxidizers, with an electrode potential of +0.56 for reduction to arsenites. (WikiPedia)
Fe3+ (PAMDB001790)
IUPAC:
iron(3+) ion
CAS: 7439-89-6
Description: The major activity of supplemental iron is in the prevention and treatment of iron deficiency anemia. Iron has putative immune-enhancing, anticarcinogenic and cognition-enhancing activities.
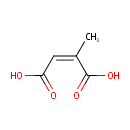
Citraconic acid (PAMDB001792)
IUPAC:
(2Z)-2-methylbut-2-enedioic acid
CAS: 498-23-7
Description: Mesaconic acid is one of several isomeric carboxylic acids obtained from citric acid. Is used as a fire retardant, recent studies revealed this acid is a competitive inhibitor of fumarate reduction.
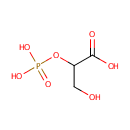
2-Phosphoglyceric acid (PAMDB001814)
IUPAC:
3-hydroxy-2-(phosphonooxy)propanoic acid
CAS: 2553-59-5
Description: 2-Phosphoglyceric acid (2PGA) is a glyceric acid which serves as the substrate in the ninth step of glycolysis. It is catalyzed by enolase into phosphoenolpyruvate (PEP), the penultimate step in the conversion of glucose to pyruvate. Enolase catalyzes the beta-elimination reaction in a stepwise manner wherein OH- is eliminated from C3 of a discrete carbanion (enolate) intermediate. This intermediate is created by removal of the proton from C2 of 2PGA by a base in the active site. (PMID: 8994873, Wikipedia)
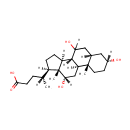
Cholic acid (PAMDB001819)
IUPAC:
(4R)-4-[(1S,2S,5R,7S,10R,11S,14R,15R,16S)-5,9,16-trihydroxy-2,15-dimethyltetracyclo[8.7.0.0?,??0??,???heptadecan-14-yl]pentanoic acid
CAS: 81-25-4
Description: Cholic acid is involved in absorption of fat and cholesterol excretion.
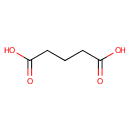
Glutaric acid (PAMDB001820)
IUPAC:
pentanedioic acid
CAS: 110-94-1
Description: Glutaric acid is a simple five-carbon linear dicarboxylic acid. It is a substrate inhibitor for glutamate decarboxylase. In many organisms it is involved in lysine degradation.
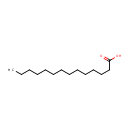
Myristic acid (PAMDB001822)
IUPAC:
tetradecanoic acid
CAS: 544-63-8
Description: Myristic acid ia a saturated 14-carbon fatty acid occurring in most animal and vegetable fats, particularly butterfat and coconut, palm, and nutmeg oils. It is used to synthesize flavor and as an ingredient in soaps and cosmetics. (From Dorland, 28th ed) Myristic acid is also commonly added to a penultimate nitrogen terminus glycine in receptor-associated kinases to confer the membrane localisation of the enzyme.