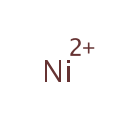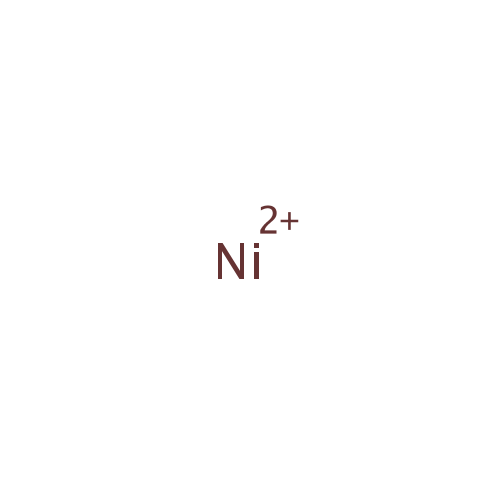|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001786 |
|---|
|
Identification |
|---|
| Name: |
Nickel |
|---|
| Description: | Nickel is a solid, silver-white, hard, malleable transition metal with an atomic number of 28. It resists corrosion even at high temperatures. It is present in many alloys in widespread use, including stainless steels. It may also be present as an impurity in any alloy. Nickel is used in the production of coins, jewellery, and nickel-cadmium batteries, and as a catalyst for the hydrogenation of liquid oils to solid fats such as oleomargarine and vegetable shortening. Nickel-containing dental alloys continue to be used successfully in the provision of various forms of dental care. Many of these alloys have applications in the construction of restorations designed to remain in clinical service for many years, including crowns, fixed bridgework, and removable partial dentures. Furthermore, nickel containing alloys find extensive application in orthodontics, including metallic brackets, arch wires, bands, springs and ligature wires. Many instruments and devises, for example, endodontic instruments also contain nickel. |
|---|
|
Structure |
|
|---|
| Synonyms: | - Carbonyl nickel powder
- Malleable nickel
- NI
- Ni(2+)
- Ni(II)
- Ni++
- Ni2+
- Nichel
- NICKEL (II) ION
- Nickel 200
- Nickel 201
- Nickel 204
- Nickel 205
- Nickel 211
- Nickel 212
- Nickel 213
- Nickel 222
- Nickel 223
- Nickel 229
- Nickel 270
- Nickel particles
- Nickel sponge
- Nickel(2+)
- Nickel, ion (Ni2+)
- Nickelous ion
- Raney alloy
- Raney nickel
|
|---|
|
Chemical Formula: |
Ni |
|---|
| Average Molecular Weight: |
58.6934 |
|---|
| Monoisotopic Molecular
Weight: |
57.935347922 |
|---|
| InChI Key: |
VEQPNABPJHWNSG-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/Ni/q+2 |
|---|
| CAS
number: |
7440-02-0 |
|---|
| IUPAC Name: | nickel(2+) ion |
|---|
|
Traditional IUPAC Name: |
nickel(2+) ion |
|---|
| SMILES: | [Ni++] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of inorganic compounds known as homogeneous transition metal compounds. These are inorganic compounds containing only metal atoms,with the largest atom being a transition metal atom. |
|---|
|
Kingdom |
Inorganic compounds |
|---|
| Super Class | Homogeneous metal compounds |
|---|
|
Class |
Homogeneous transition metal compounds |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Homogeneous transition metal compounds |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Homogeneous transition metal
- Inorganic nickel compound
- Acyclic compound
|
|---|
| Molecular Framework |
Acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 2 |
|---|
|
Melting point: |
1455 °C |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Biego GH, Joyeux M, Hartemann P, Debry G: Daily intake of essential minerals and metallic micropollutants from foods in France. Sci Total Environ. 1998 Jun 30;217(1-2):27-36. Pubmed: 9695171
- Hostynek JJ: Sensitization to nickel: etiology, epidemiology, immune reactions, prevention, and therapy. Rev Environ Health. 2006 Oct-Dec;21(4):253-80. Pubmed: 17243350
- Nielsen FH: How should dietary guidance be given for mineral elements with beneficial actions or suspected of being essential? J Nutr. 1996 Sep;126(9 Suppl):2377S-2385S. Pubmed: 8811801
- Setcos JC, Babaei-Mahani A, Silvio LD, Mjor IA, Wilson NH: The safety of nickel containing dental alloys. Dent Mater. 2006 Dec;22(12):1163-8. Epub 2006 Jan 6. Pubmed: 16405986
- Uthus EO, Seaborn CD: Deliberations and evaluations of the approaches, endpoints and paradigms for dietary recommendations of the other trace elements. J Nutr. 1996 Sep;126(9 Suppl):2452S-2459S. Pubmed: 8811811
|
|---|
| Synthesis Reference: |
Lightner, David A.; Crist, B. Vincent; Kalyanam, Nagabushanam; May, Leslie M.; Jackman, Dennis E. The octant rule. 15. Antioctant effects: synthesis and circular dichroism of 2-exo- and 2-endo-alkylbicyclo[2.2.1]heptan-7-ones and bicyclo[3.2.1]octan-8-one |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|