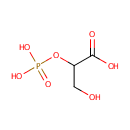
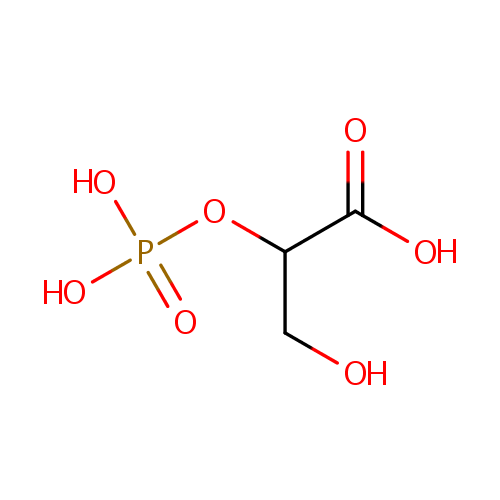
| References: |
- Comi GP, Fortunato F, Lucchiari S, Bordoni A, Prelle A, Jann S, Keller A, Ciscato P, Galbiati S, Chiveri L, Torrente Y, Scarlato G, Bresolin N: Beta-enolase deficiency, a new metabolic myopathy of distal glycolysis. Ann Neurol. 2001 Aug;50(2):202-7. Pubmed: 11506403
- Fujii H: [Red cell glycolytic intermediates] Nippon Rinsho. 1995 Mar;53 Su Pt 2:234-8. Pubmed: 8753225
- Hakobyan D, Nazaryan K: Investigation of interaction between enolase and phosphoglycerate mutase using molecular dynamics simulation. J Biomol Struct Dyn. 2006 Jun;23(6):625-34. Pubmed: 16615808
- Mulquiney PJ, Bubb WA, Kuchel PW: Model of 2,3-bisphosphoglycerate metabolism in the human erythrocyte based on detailed enzyme kinetic equations: in vivo kinetic characterization of 2,3-bisphosphoglycerate synthase/phosphatase using 13C and 31P NMR. Biochem J. 1999 Sep 15;342 Pt 3:567-80. Pubmed: 10477268
- Nakayama Y, Kinoshita A, Tomita M: Dynamic simulation of red blood cell metabolism and its application to the analysis of a pathological condition. Theor Biol Med Model. 2005 May 9;2(1):18. Pubmed: 15882454
- Reed, G. H., Poyner, R. R., Larsen, T. M., Wedekind, J. E., Rayment, I. (1996). "Structural and mechanistic studies of enolase." Curr Opin Struct Biol 6:736-743. Pubmed: 8994873
- Reher M, Bott M, Schonheit P: Characterization of glycerate kinase (2-phosphoglycerate forming), a key enzyme of the nonphosphorylative Entner-Doudoroff pathway, from the thermoacidophilic euryarchaeon Picrophilus torridus. FEMS Microbiol Lett. 2006 Jun;259(1):113-9. Pubmed: 16684110
- Tsujino S, Shanske S, Sakoda S, Toscano A, DiMauro S: Molecular genetic studies in muscle phosphoglycerate mutase (PGAM-M) deficiency. Muscle Nerve. 1995;3:S50-3. Pubmed: 7603528
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Wang Y, Wei Z, Liu L, Cheng Z, Lin Y, Ji F, Gong W: Crystal structure of human B-type phosphoglycerate mutase bound with citrate. Biochem Biophys Res Commun. 2005 Jun 17;331(4):1207-15. Pubmed: 15883004
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|