|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001819 |
|---|
|
Identification |
|---|
| Name: |
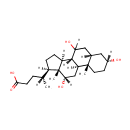
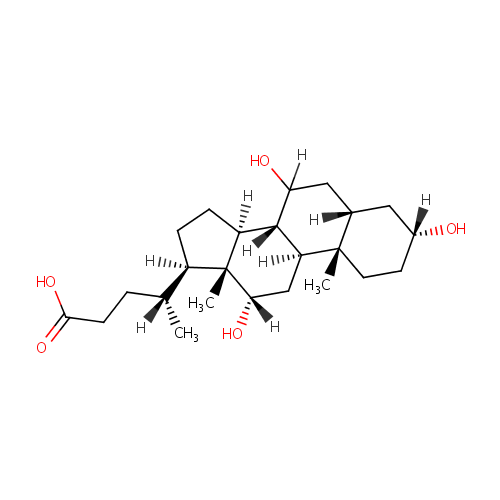
Cholic acid |
|---|
| Description: | Cholic acid is involved in absorption of fat and cholesterol excretion. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 17b-[1-Methyl-3-carboxypropyl]etiocholane-3a,7a,12a-triol
- 3a,7a,12a-Trihydroxy-5b-cholan-24-oate
- 3a,7a,12a-Trihydroxy-5b-cholan-24-oic acid
- 3a,7a,12a-Trihydroxy-5b-cholanate
- 3a,7a,12a-Trihydroxy-5b-cholanic acid
- 3a,7a,12a-Trihydroxy-5b-cholanoate
- 3a,7a,12a-Trihydroxy-5b-cholanoic acid
- 3a,7a,12a-Trihydroxy-b-cholanate
- 3a,7a,12a-Trihydroxy-b-cholanic acid
- 3a,7a,12a-Trihydroxy-beta-cholanate
- 3a,7a,12a-Trihydroxy-beta-cholanic acid
- 3a,7a,12a-Trihydroxy-β-cholanate
- 3a,7a,12a-Trihydroxy-β-cholanic acid
- 3a,7a,12a-Trihydroxycholanate
- 3a,7a,12a-Trihydroxycholanic acid
- 5b-Cholanate-3a,7a,12a-triol
- 5b-Cholanic acid-3a,7a,12a-triol
- 5b-Cholate
- 5b-Cholic acid
- Cholalate
- Cholalic acid
- Cholalin
- Cholate
- Colalin
|
|---|
|
Chemical Formula: |
C24H40O5 |
|---|
| Average Molecular Weight: |
408.5714 |
|---|
| Monoisotopic Molecular
Weight: |
408.28757439 |
|---|
| InChI Key: |
BHQCQFFYRZLCQQ-IATNSQEUSA-N |
|---|
| InChI: | InChI=1S/C24H40O5/c1-13(4-7-21(28)29)16-5-6-17-22-18(12-20(27)24(16,17)3)23(2)9-8-15(25)10-14(23)11-19(22)26/h13-20,22,25-27H,4-12H2,1-3H3,(H,28,29)/t13-,14+,15-,16-,17+,18+,19?,20+,22+,23+,24-/m1/s1 |
|---|
| CAS
number: |
81-25-4 |
|---|
| IUPAC Name: | (4R)-4-[(1S,2S,5R,7S,10R,11S,14R,15R,16S)-5,9,16-trihydroxy-2,15-dimethyltetracyclo[8.7.0.0?,??0??,???heptadecan-14-yl]pentanoic acid |
|---|
|
Traditional IUPAC Name: |
(4R)-4-[(1S,2S,5R,7S,10R,11S,14R,15R,16S)-5,9,16-trihydroxy-2,15-dimethyltetracyclo[8.7.0.0?,??0??,???heptadecan-14-yl]pentanoic acid |
|---|
| SMILES: | [H][C@@](C)(CCC(O)=O)[C@@]1([H])CC[C@@]2([H])[C@]3([H])C([H])(O)C[C@]4([H])C[C@]([H])(O)CC[C@]4(C)[C@@]3([H])C[C@]([H])(O)[C@]12C |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as trihydroxy bile acids, alcohols and derivatives. These are prenol lipids structurally characterized by a bile acid or alcohol which bears three hydroxyl groups. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Steroids and steroid derivatives |
|---|
| Sub Class | Bile acids, alcohols and derivatives |
|---|
|
Direct Parent |
Trihydroxy bile acids, alcohols and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Trihydroxy bile acid, alcohol, or derivatives
- 7-hydroxysteroid
- 3-alpha-hydroxysteroid
- Hydroxysteroid
- 12-hydroxysteroid
- 3-hydroxysteroid
- Cyclic alcohol
- Secondary alcohol
- Polyol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework |
Aliphatic homopolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
197-201 °C |
|---|
| Experimental Properties: |
| Property | Value | Source |
|---|
| Water Solubility: | 0.175 mg/mL [YALKOWSKY,SH & DANNENFELSER,RM (1992)] | PhysProp | | LogP: | 2.02 [RODA,A ET AL. (1990)] | PhysProp |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|