Search Results for compounds
Searching compounds for
returned 4373 results.
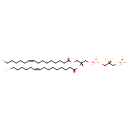
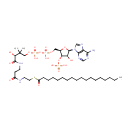
PGP(16:1(9Z)/16:1(9Z)) (PAMDB001679)
IUPAC:
[(2R)-3-({[(2R)-2,3-bis[(9Z)-hexadec-9-enoyloxy]propoxy](hydroxy)phosphoryl}oxy)-2-hydroxypropoxy]phosphonic acid
CAS: Not Available
Description: PGP(16:1(9Z)/16:1(9Z)) belongs to the class of glycerophosphoglycerophosphates, also called phosphatidylglycerophosphates (PGPs). These lipids contain a common glycerophosphate skeleton linked to at least one fatty acyl chain and a glycero-3-phosphate moiety. As is the case with diacylglycerols, phosphatidylglycerophosphates can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. PGP(16:1(9Z)/16:1(9Z)), in particular, consists of two 9Z-hexadecenoyl chains at positions C-1 and C-2. In Pseudomonas aeruginosa, PGPs can be found in the cytoplasmic membrane. The are synthesized by the addition of glycerol 3-phosphate to a CDP-diacylglycerol. In turn, PGPs are dephosphorylated to Phosphatidylglycerols (PGs) by the enzyme Phosphatidylglycerophosphatase.
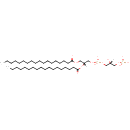
PGP(18:0/18:0) (PAMDB001680)
IUPAC:
[(2R)-3-({[(2R)-2,3-bis(octadecanoyloxy)propoxy](hydroxy)phosphoryl}oxy)-2-hydroxypropoxy]phosphonic acid
CAS: Not Available
Description: PGP(18:0/18:0) belongs to the class of glycerophosphoglycerophosphates, also called phosphatidylglycerophosphates (PGPs). These lipids contain a common glycerophosphate skeleton linked to at least one fatty acyl chain and a glycero-3-phosphate moiety. As is the case with diacylglycerols, phosphatidylglycerophosphates can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. PGP(18:0/18:0), in particular, consists of two octadecanoyl chains at positions C-1 and C-2. In Pseudomonas aeruginosa, PGPs can be found in the cytoplasmic membrane. The are synthesized by the addition of glycerol 3-phosphate to a CDP-diacylglycerol. In turn, PGPs are dephosphorylated to Phosphatidylglycerols (PGs) by the enzyme Phosphatidylglycerophosphatase.
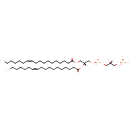
PGP(18:1(11Z)/18:1(11Z)) (PAMDB001681)
IUPAC:
[(2R)-3-({[(2R)-2,3-bis[(11Z)-octadec-11-enoyloxy]propoxy](hydroxy)phosphoryl}oxy)-2-hydroxypropoxy]phosphonic acid
CAS: Not Available
Description: PGP(18:1(11Z)/18:1(11Z)) belongs to the class of glycerophosphoglycerophosphates, also called phosphatidylglycerophosphates (PGPs). These lipids contain a common glycerophosphate skeleton linked to at least one fatty acyl chain and a glycero-3-phosphate moiety. As is the case with diacylglycerols, phosphatidylglycerophosphates can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. PGP(18:1(11Z)/18:1(11Z)), in particular, consists of two 11Z-octadecenoyl chains at positions C-1 and C-2. In Pseudomonas aeruginosa, PGPs can be found in the cytoplasmic membrane. The are synthesized by the addition of glycerol 3-phosphate to a CDP-diacylglycerol. In turn, PGPs are dephosphorylated to Phosphatidylglycerols (PGs) by the enzyme Phosphatidylglycerophosphatase.
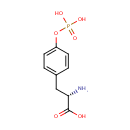
Phosphotyrosine (PAMDB001683)
IUPAC:
(2S)-2-amino-3-[4-(phosphonooxy)phenyl]propanoic acid
CAS: 21820-51-9
Description: Phosphotyrosine is a tyrosine (an amino acid) with a phosphate attached to its aromatic ring. Tyrosine (abbreviated as Tyr or Y) is one of the 22 amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. (Wikipedia) Tyrosine phosphorylation and dephosphorylation plays a role in cellular signal transduction and possibly in cell growth control. (PubChem) In Pseudomonas aeruginosa, phosphotyrosine can act as a noncompetitive inhibitor of the enzyme biosynthetic ornithine decarboxylase. (EcoCyc)
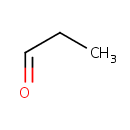
Propanal (PAMDB001684)
IUPAC:
propanal
CAS: 123-38-6
Description: Propanal or propionaldehyde is the aldehyde of the 3 carbon propyl group. It has a chemical formula of CH3CH2CHO, and is a structural isomer of propanone. At room temperature, it is a colourless liquid with a slightly irritating, fruity odour.; It is principally used as a precursor to trimethylolethane (CH3C(CH2OH)3) through a condensation reaction with formaldehyde; this triol is an important intermediate in the production of alkyd resins. Other applications include reduction to propanol and oxidation to propionic acid.
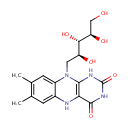
Reduced riboflavin (PAMDB001686)
IUPAC:
7,8-dimethyl-10-[(2S,3S,4R)-2,3,4,5-tetrahydroxypentyl]-1H,2H,3H,4H,5H,10H-benzo[g]pteridine-2,4-dione
CAS: Not Available
Description: Reduced riboflavin is a member of the chemical class known as Quinoxalines. These are compounds containing a quinoxaline moiety, a bicyclic heterocycle made up of a benzene ring fused to a pyrazine ring. Compared to riboflavin, reduced riboflavin has one extra hydrogen attached to an oxygen on its ring structure. Riboflavin, also known as vitamin B2, is the central component of the cofactors FAD and FMN, and is therefore required by all flavoproteins. As such, vitamin B2 is required for a wide variety of cellular processes. It plays a key role in energy metabolism, and for the metabolism of fats, ketone bodies, carbohydrates, and proteins. The reduced form, which occurs in metabolism along with the oxidized form, is colorless. (Wikipedia)
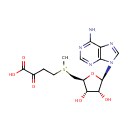
S-Adenosyl-4-methylthio-2-oxobutanoate (PAMDB001688)
IUPAC:
{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl}(3-carboxy-3-oxopropyl)methylsulfanium
CAS: Not Available
Description: S-adenosyl-4-methylthio-2-oxobutanoate is a member of the chemical class known as Purine Nucleosides and Analogues. These are compounds comprising a purine base attached to a sugar.
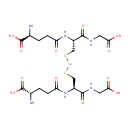
Selenodiglutathione (PAMDB001689)
IUPAC:
(2S)-2-amino-4-{[(1R)-2-[({[(2R)-2-[(4S)-4-amino-4-carboxybutanamido]-2-[(carboxymethyl)carbamoyl]ethyl]sulfanyl}selanyl)sulfanyl]-1-[(carboxymethyl)carbamoyl]ethyl]carbamoyl}butanoic acid
CAS: 33944-90-0
Description: Selenodiglutathione is a member of the chemical class known as Peptides. These are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another. Selenium compounds like selenite (SeO3(2-) may form a covalent adduct with glutathione (GSH) in the form of selenodiglutathione (GS-Se-SG), which is assumed to be important in the metabolism of selenium. GS-Se-SG to be a very efficient oxidant of reduced thioredoxin from Pseudomonas aeruginosa [PMID:1569062]
Stearoyl-CoA (PAMDB001690)
IUPAC:
{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({hydroxy[(3R)-3-hydroxy-2,2-dimethyl-3-[(2-{[2-(octadecanoylsulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]propoxy]phosphoryl}oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid
CAS: 362-66-3
Description: Stearoyl-CoA is derived from stearic acid, which is a saturated fatty acid consisting of 18 carbons. Stearoyl-CoA is a long-chain acyl CoA ester that acts as an intermediate metabolite in the biosynthesis of monounsaturated fatty acids. Stearoyl-CoA is converted into oleoyl-CoA and then used as a major substrate for the synthesis of various kinds of lipids including phospholipids, triglycerides, cholesteryl esters and wax esters. There is growing recognition that acyl-CoA esters could act as signaling molecules in cellular metabolism. (HMDB)
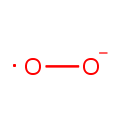
Superoxide anion (PAMDB001691)
IUPAC:
oxidanidyloxidanyl
CAS: 11062-77-4
Description: The superoxide ion, also known by the obsolete name hyperoxide, is a compound that contains the superoxide anion with the chemical formula O2-. The systematic name of the anion is dioxide. The superoxide anion is particularly important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature. With one unpaired electron, the superoxide ion is a free radical, and, like dioxygen, it is paramagnetic.; Because superoxide is toxic, nearly all organisms living in the presence of oxygen contain isoforms of the superoxide scavenging enzyme, superoxide dismutase, or SOD. SOD is an extremely efficient enzyme; it catalyzes the neutralization of superoxide nearly as fast as the two can diffuse together spontaneously in solution. Other proteins, which can be both oxidized and reduced by superoxide, have weak SOD-like activity (e.g. hemoglobin). Genetic inactivation ('knockout') of SOD produces deleterious phenotypes in organisms ranging from bacteria to mice and have provided important clues as to the mechanisms of toxicity of superoxide in vivo.; Superoxide is the anionic form O2. It is important as the product of the one-electron reduction of dioxygen (oxygen gas), which occurs widely in nature. With one unpaired electron, the superoxide ion is a free radical. It is also paramagnetic. The biological toxicity of superoxide is due to its capacity to inactivate iron-sulfur cluster containing enzymes (which are critical in a wide variety of metabolic pathways), thereby liberating free iron in the cell, which can undergo fenton-chemistry and generate the highly reactive hydroxyl radical. In its HO2 form, superoxide can also initiate lipid peroxidation of polyunsaturated fatty acids. It also reacts with carbonyl compounds and halogenated carbons to create toxic peroxy radicals. As such, superoxide is a main cause of oxidative stress.; Highly reactive compounds produced when oxygen is reduced by a single electron. In biological systems, they may be generated during the normal catalytic function of a number of enzymes and during the oxidation of hemoglobin to Methemoglobin.; Because superoxide is toxic, nearly all organisms living in the presence of oxygen contain isoforms of the superoxide scavenging enzyme, superoxide dismutase, or SOD. SOD is an extremely efficient enzyme; it catalyzes the neutralization of superoxide nearly as fast as the two can diffuse together spontaneously in solution. With one unpaired electron, the superoxide ion is a free radical and therefore paramagnetic.; In living organisms, superoxide dismutase protects the cell from the deleterious effects of superoxides.