|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001683 |
|---|
|
Identification |
|---|
| Name: |
Phosphotyrosine |
|---|
| Description: | Phosphotyrosine is a tyrosine (an amino acid) with a phosphate attached to its aromatic ring. Tyrosine (abbreviated as Tyr or Y) is one of the 22 amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. (Wikipedia) Tyrosine phosphorylation and dephosphorylation plays a role in cellular signal transduction and possibly in cell growth control. (PubChem) In Pseudomonas aeruginosa, phosphotyrosine can act as a noncompetitive inhibitor of the enzyme biosynthetic ornithine decarboxylase. (EcoCyc) |
|---|
|
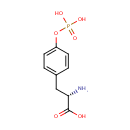
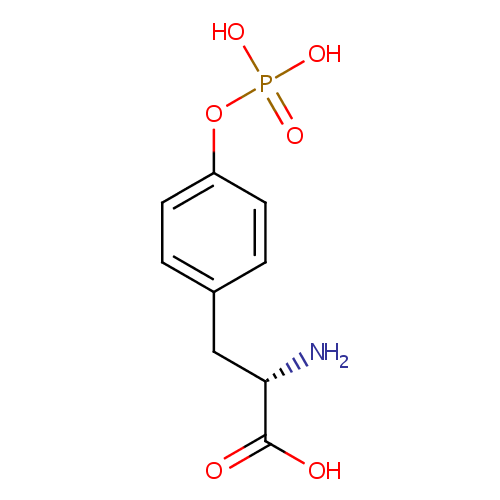
Structure |
|
|---|
| Synonyms: | - (S)-2-amino-3-(4-hydroxyphenyl)propanoate 4'-phosphate
- (S)-2-amino-3-(4-hydroxyphenyl)propanoic acid 4'-phosphate
- (S)-2-amino-3-(4-Hydroxyphenyl)propanoic acid 4'-phosphoric acid
- L-3-(4-Hydroxyphenyl)alanine 4'-phosphate
- L-3-(4-Hydroxyphenyl)alanine 4'-phosphoric acid
- L-Phosphotyrosine
- L-Tyrosine, dihydrogen phosphate (ester)
- L-Tyrosine, dihydrogen phosphoric acid (ester)
- L-Tyrosine-O-phosphate
- L-Tyrosine-O-phosphoric acid
- O-Phospho-L-tyrosine
- O-Phosphono-L-tyrosine
- O-Phosphotyrosine
- O4'-phospho-L-tyrosine
- O4-phosphono-L-tyrosine
- O4-phosphotyrosine
- Phospho-L-tyrosine
- Phosphonotyrosine
- Phosphotyrosine
- Phosphotyrosine (pY)
- Tyrosine O-phosphate
- Tyrosine O-phosphoric acid
- Tyrosine phosphate
- Tyrosine phosphoric acid
|
|---|
|
Chemical Formula: |
C9H12NO6P |
|---|
| Average Molecular Weight: |
261.1684 |
|---|
| Monoisotopic Molecular
Weight: |
261.040223633 |
|---|
| InChI Key: |
DCWXELXMIBXGTH-QMMMGPOBSA-N |
|---|
| InChI: | InChI=1S/C9H12NO6P/c10-8(9(11)12)5-6-1-3-7(4-2-6)16-17(13,14)15/h1-4,8H,5,10H2,(H,11,12)(H2,13,14,15)/t8-/m0/s1 |
|---|
| CAS
number: |
21820-51-9 |
|---|
| IUPAC Name: | (2S)-2-amino-3-[4-(phosphonooxy)phenyl]propanoic acid |
|---|
|
Traditional IUPAC Name: |
phosphonotyrosine |
|---|
| SMILES: | N[C@@H](CC1=CC=C(OP(O)(O)=O)C=C1)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as phenylpropanoic acids. These are compounds with a structure containing a benzene ring conjugated to a propanoic acid. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
|
Class |
Phenylpropanoic acids |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Phenylpropanoic acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 3-phenylpropanoic-acid
- L-alpha-amino acid
- Amphetamine or derivatives
- Alpha-amino acid or derivatives
- Alpha-amino acid
- Aralkylamine
- Amino fatty acid
- Fatty acyl
- Benzenoid
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic phosphate
- Monocyclic benzene moiety
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kataoka H, Nakai K, Makita M: Increase of phosphotyrosine levels in mouse urine and liver during liver regeneration after partial hepatectomy. Biochem Biophys Res Commun. 1994 Jun 15;201(2):909-16. Pubmed: 7516161
- Kataoka, H., Nakai, K., Katagiri, Y., Makita, M. (1993). "Analysis of free and bound O-phosphoamino acids in urine by gas chromatography with flame photometric detection." Biomed Chromatogr 7:184-188. Pubmed: 7693088
- Munoz GE, Arenas-Diaz G, Marshall SH: Exogenously added free phosphotyrosine induces aggregation of circulating platelets in rabbits. Cell Mol Biol (Noisy-le-grand). 1992 Nov;38(7):719-22. Pubmed: 1282059
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|