|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001691 |
|---|
|
Identification |
|---|
| Name: |
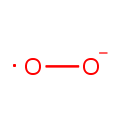
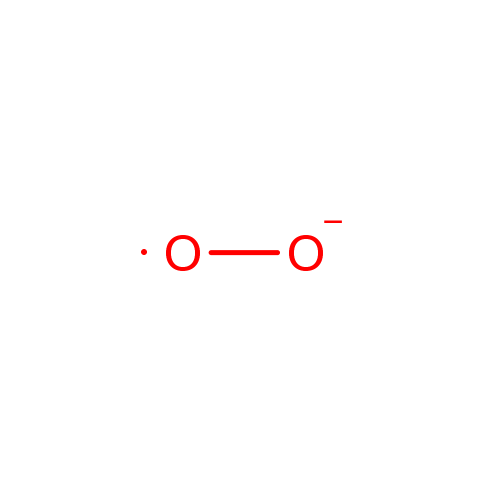
Superoxide anion |
|---|
| Description: | The superoxide ion, also known by the obsolete name hyperoxide, is a compound that contains the superoxide anion with the chemical formula O2-. The systematic name of the anion is dioxide. The superoxide anion is particularly important as the product of the one-electron reduction of dioxygen O2, which occurs widely in nature. With one unpaired electron, the superoxide ion is a free radical, and, like dioxygen, it is paramagnetic.; Because superoxide is toxic, nearly all organisms living in the presence of oxygen contain isoforms of the superoxide scavenging enzyme, superoxide dismutase, or SOD. SOD is an extremely efficient enzyme; it catalyzes the neutralization of superoxide nearly as fast as the two can diffuse together spontaneously in solution. Other proteins, which can be both oxidized and reduced by superoxide, have weak SOD-like activity (e.g. hemoglobin). Genetic inactivation ('knockout') of SOD produces deleterious phenotypes in organisms ranging from bacteria to mice and have provided important clues as to the mechanisms of toxicity of superoxide in vivo.; Superoxide is the anionic form O2. It is important as the product of the one-electron reduction of dioxygen (oxygen gas), which occurs widely in nature. With one unpaired electron, the superoxide ion is a free radical. It is also paramagnetic. The biological toxicity of superoxide is due to its capacity to inactivate iron-sulfur cluster containing enzymes (which are critical in a wide variety of metabolic pathways), thereby liberating free iron in the cell, which can undergo fenton-chemistry and generate the highly reactive hydroxyl radical. In its HO2 form, superoxide can also initiate lipid peroxidation of polyunsaturated fatty acids. It also reacts with carbonyl compounds and halogenated carbons to create toxic peroxy radicals. As such, superoxide is a main cause of oxidative stress.; Highly reactive compounds produced when oxygen is reduced by a single electron. In biological systems, they may be generated during the normal catalytic function of a number of enzymes and during the oxidation of hemoglobin to Methemoglobin.; Because superoxide is toxic, nearly all organisms living in the presence of oxygen contain isoforms of the superoxide scavenging enzyme, superoxide dismutase, or SOD. SOD is an extremely efficient enzyme; it catalyzes the neutralization of superoxide nearly as fast as the two can diffuse together spontaneously in solution. With one unpaired electron, the superoxide ion is a free radical and therefore paramagnetic.; In living organisms, superoxide dismutase protects the cell from the deleterious effects of superoxides. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (O2)
- Dioxidanidyl
- Dioxide(1)
- Dioxide(1-)
- Hyperoxid
- Hyperoxide
- O2
- O2 free radical
- O2-
- O2.-
- O2-
- Peroxide radical
- Superoxide
- Superoxide anion
- Superoxide anion radical
- Superoxide radical
- Superoxide radical anion
- Superoxyde
|
|---|
|
Chemical Formula: |
O2 |
|---|
| Average Molecular Weight: |
31.9988 |
|---|
| Monoisotopic Molecular
Weight: |
31.989829244 |
|---|
| InChI Key: |
OUUQCZGPVNCOIJ-UHFFFAOYSA-M |
|---|
| InChI: | InChI=1S/HO2/c1-2/h1H/p-1 |
|---|
| CAS
number: |
11062-77-4 |
|---|
| IUPAC Name: | oxidanidyloxidanyl |
|---|
|
Traditional IUPAC Name: |
superoxide |
|---|
| SMILES: | [O][O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of inorganic compounds known as non-metal superoxides. These are inorganic non-metallic compoundscontaining a superoxide as its largest oxoanion. |
|---|
|
Kingdom |
Inorganic compounds |
|---|
| Super Class | Homogeneous non-metal compounds |
|---|
|
Class |
Non-metal oxoanionic compounds |
|---|
| Sub Class | Non-metal superoxides |
|---|
|
Direct Parent |
Non-metal superoxides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Non-metal superoxide
- Inorganic oxide
- Acyclic compound
|
|---|
| Molecular Framework |
Acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|