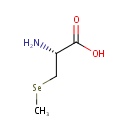Search Results for compounds
Searching compounds for
returned 4373 results.
UDP-N-Acetylmuramoyl-L-alanyl-D-glutamyl-meso-2,6-diaminoheptanedioate-D-alanine (PAMDB001419)
IUPAC:
1-[(2R,3R,4S,5R)-5-{[({[(2R,3R,4R,5S,6R)-4-[(1R)-1-{[(1S)-1-{[(1R)-3-{[(1S,5R)-5-amino-5-carboxy-1-{[(1R)-1-carboxyethyl]-C-hydroxycarbonimidoyl}pentyl]carboximidato}-1-carboxypropyl]-C-hydroxycarbonimidoyl}ethyl]-C-hydroxycarbonimidoyl}ethoxy]-5-hydroxy-6-(hydroxymethyl)-3-[(1-oxidoethylidene)amino]oxan-2-yl phosphonato]oxy}phosphinato)oxy]methyl}-3,4-dihydroxyoxolan-2-yl]-4-hydroxy-2-oxo-2,5-dihydro-1???pyrimidine-1,5-bis(ylium)
CAS: Not Available
Description: UDP-N-acetylmuramoyl-L-alanyl-D-glutamyl-meso-2,6-diaminoheptanedioate-D-alanine is a member of the chemical class known as Peptides. These are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another. It is a key component of peptidoglycan synthesis. The peptidoglycan synthesis pathway starts at the cytoplasm, where in six steps the peptidoglycan precursor a UDP-N-acetylmuramoyl-pentapeptide is synthesized. This precursor is then attached to the memberane acceptor all-trans-undecaprenyl phosphate, generating a N-acetylmuramoyl-pentapeptide-diphosphoundecaprenol, also known as lipid I. Another transferase then adds UDP-N-acetyl-alpha-D-glucosamine, yielding the complete monomeric unit a lipid , also known as lipid . This final lipid intermediate is transferred through the membrane. The peptidoglycan monomers are then polymerized on the outside surface by glycosyltransferases, which form the linear glycan chains, and transpeptidases, which catalyze the formation of peptide crosslinks.
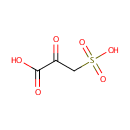
3-Sulfopyruvic acid (PAMDB001422)
IUPAC:
2-oxo-3-sulfopropanoic acid
CAS: 98022-26-5
Description: 3-Sulfopyruvic acid is the product of the transamination of cysteinesulfonate in a reaction catalyzed by aspartate aminotransferase. 3-sulfopyruvic acid is stable and is reduced by malate dehydrogenase to beta-sulfolactate. Cysteinesulfonate, 3-sulfopyruvic acid, and beta-sulfolactate are reversibly interconverted in vivo.
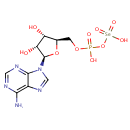
Adenylylselenate (PAMDB001423)
IUPAC:
[({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]selenonic acid
CAS: Not Available
Description: Adenylylselenate is an intermediate in selenoamino acid metabolism. Adenylylselenate is produced from selenate via the enzyme sulfate adenylyltransferase [EC:2.7.7.4] and then converted to selenite via the enzyme adenylylsulfate reductase [EC:1.8.99.2].
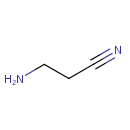
beta-Aminopropionitrile (PAMDB001424)
IUPAC:
3-aminopropanenitrile
CAS: 151-18-8
Description: Beta-Aminopropionitrile is a toxic amino-acid derivative. It is an metabolite in beta-alanine metabolism and converted to beta-alanine. It is also an intermediate in cyanoamino acid metablism. (KEGG)
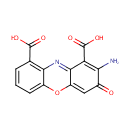
Cinnavalininate (PAMDB001425)
IUPAC:
2-amino-3-oxo-3H-phenoxazine-1,9-dicarboxylic acid
CAS: Not Available
Description: Cinnavalininate is an intermediate in the tryptophan metabolic pathway [Kegg: C05640]. It is generated from 3-hydroxyanthranilate via the enzyme catalase (EC:1.11.1.6).
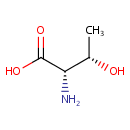
L-Allothreonine (PAMDB001426)
IUPAC:
(2S,3S)-2-amino-3-hydroxybutanoic acid
CAS: 24830-94-2
Description: Allothreonine is the substrate of the enzyme Serine hydroxymethyltransferase1 (SHMT, EC 2.1.2.1). SHMT uses pyridoxal 5'-phosphate (PLP) and tetrahydropteroylglutamate (H4PteGlu) as coenzymes and catalyzes the reversible interconversion of serine and glycine. In addition to these physiological reactions, SHMT also catalyzes, in the absence of H4PteGlu, the retroaldol cleavage of several 3-hydroxyamino acids, such as allothreonine.
Se-Methylselenocysteine (PAMDB001427)
IUPAC:
(2R)-2-amino-3-(methylselanyl)propanoic acid
CAS: 26046-90-2
Description: Se-Methylselenocysteine (SeMSC) is a naturally occurring seleno-amino acid that is synthesized by plants such as garlic, astragalus, onions and broccoli. SeMSC is an intermediate in selenoamino acid metabolism. Unlike selenomethionine, which is incorporated into proteins in place of methionine, SeMSC is not incorporated into any proteins, thereby being fully available for the synthesis of selenium-containing enzymes such as glutathione peroxidase.
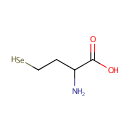
Selenohomocysteine (PAMDB001428)
IUPAC:
2-amino-4-selanylbutanoic acid
CAS: 29412-93-9
Description: Selenohomocysteine is the precursor of selenocysteine, which is synthesized by catalysis of cystathionine beta-synthase (EC 4.2.1.22) and cystathionine gamma-lyase (EC 4.4.1.1). Selenohomocysteine (lactone) has been found to be a competitive and irreversible inhibitor of lysyl oxidase. L-selenohomocysteine also can serve as a substituent donor in the beta-replacement reaction to yield selenocystathionine. (PMID: 10609891, 9405445, 6456763, 3338973)
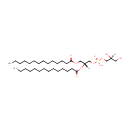
PG(14:0/14:0) (PAMDB001429)
IUPAC:
[(2R)-2,3-bis(tetradecanoyloxy)propoxy][(2S)-2,3-dihydroxypropoxy]phosphinic acid
CAS: Not Available
Description: PG(14:0/14:0) is a phosphatidylglycerol. Phosphatidylglycerols consist of a glycerol 3-phosphate backbone esterified to either saturated or unsaturated fatty acids on carbons 1 and 2. As is the case with diacylglycerols, phosphatidylglycerols can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 positions. PG(14:0/14:0), in particular, consists of two tetradecanoyl chains at positions C-1 and C-2. In Pseudomonas aeruginosa glycerophospholipid metabolism, phosphatidylglycerol is formed from phosphatidic acid (1,2-diacyl-sn-glycerol 3-phosphate) by a sequence of enzymatic reactions that proceeds via two intermediates, cytidine diphosphate diacylglycerol (CDP-diacylglycerol) and phosphatidylglycerophosphate (PGP, a phosphorylated phosphatidylglycerol). Phosphatidylglycerols, along with CDP-diacylglycerol, also serve as precursor molecules for the synthesis of cardiolipin, a phospholipid found in membranes.
PE(17:0/16:0) (PAMDB001430)
IUPAC:
(2-aminoethoxy)[(2R)-3-(heptadecanoyloxy)-2-(hexadecanoyloxy)propoxy]phosphinic acid
CAS: Not Available
Description: PE(17:0/16:0) is a phosphatidylethanolamine. It is a glycerophospholipid in which a phosphorylethanolamine moiety occupies a glycerol substitution site. As is the case with diacylglycerols, glycerophosphoethanolamines can have many different combinations of fatty acids of varying lengths and saturation attached to the C-1 and C-2 atoms. PE(17:0/16:0), in particular, consists of one heptadecanoyl chain to the C-1 atom, and one hexadecanoyl to the C-2 atom. While most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone, the fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. PEs are neutral zwitterions at physiological pH. They mostly have palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2. PE synthesis can occur via two pathways. The first requires that ethanolamine be activated by phosphorylation and then coupled to CDP. The ethanolamine is then transferred from CDP-ethanolamine to phosphatidic acid to yield PE. The second involves the decarboxylation of PS.