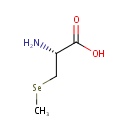|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001427 |
|---|
|
Identification |
|---|
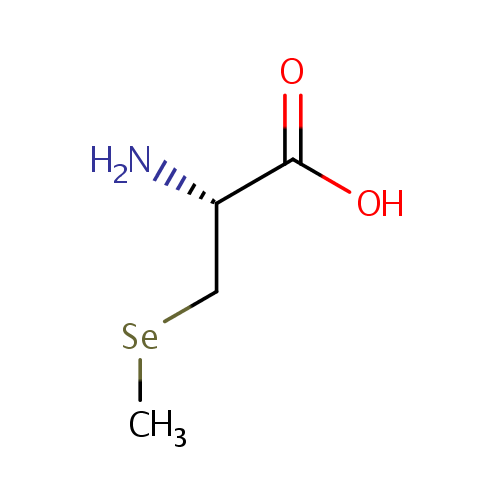
| Name: |
Se-Methylselenocysteine |
|---|
| Description: | Se-Methylselenocysteine (SeMSC) is a naturally occurring seleno-amino acid that is synthesized by plants such as garlic, astragalus, onions and broccoli. SeMSC is an intermediate in selenoamino acid metabolism. Unlike selenomethionine, which is incorporated into proteins in place of methionine, SeMSC is not incorporated into any proteins, thereby being fully available for the synthesis of selenium-containing enzymes such as glutathione peroxidase. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 3-(Methylseleno)-L-Alanine
- Methylselenocysteine
- Se-Methyl-L-selenocysteine
- Selenium-methylselenocystine
|
|---|
|
Chemical Formula: |
C4H9NO2Se |
|---|
| Average Molecular Weight: |
182.08 |
|---|
| Monoisotopic Molecular
Weight: |
182.979850365 |
|---|
| InChI Key: |
XDSSPSLGNGIIHP-VKHMYHEASA-N |
|---|
| InChI: | InChI=1S/C4H9NO2Se/c1-8-2-3(5)4(6)7/h3H,2,5H2,1H3,(H,6,7)/t3-/m0/s1 |
|---|
| CAS
number: |
26046-90-2 |
|---|
| IUPAC Name: | (2R)-2-amino-3-(methylselanyl)propanoic acid |
|---|
|
Traditional IUPAC Name: |
methylselenocysteine |
|---|
| SMILES: | C[Se]C[C@H](N)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as d-alpha-amino acids. These are alpha amino acids which have the D-configuration of the alpha-carbon atom. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
D-alpha-amino acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- D-alpha-amino acid
- Selenoether
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Hydrocarbon derivative
- Primary amine
- Organoselenium compound
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Yin MB, Li ZR, Cao S, Durrani FA, Azrak RG, Frank C, Rustum YM: Enhanced 7-ethyl-10-hydroxycamptothecin (SN-38) lethality by methylselenocysteine is associated with Chk2 phosphorylation at threonine-68 and down-regulation of Cdc6 expression. Mol Pharmacol. 2004 Jul;66(1):153-60. Pubmed: 15213307
|
|---|
| Synthesis Reference: |
Spallholz, Julian E.; Reid, Ted W.; Walkup, Robert D. A method of using synthetic L-Se-methylselenocysteine as a nutriceutical. Eur. Pat. Appl. (2002), 21 pp. |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|