|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001425 |
|---|
|
Identification |
|---|
| Name: |
Cinnavalininate |
|---|
| Description: | Cinnavalininate is an intermediate in the tryptophan metabolic pathway [Kegg: C05640]. It is generated from 3-hydroxyanthranilate via the enzyme catalase (EC:1.11.1.6). |
|---|
|
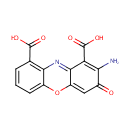
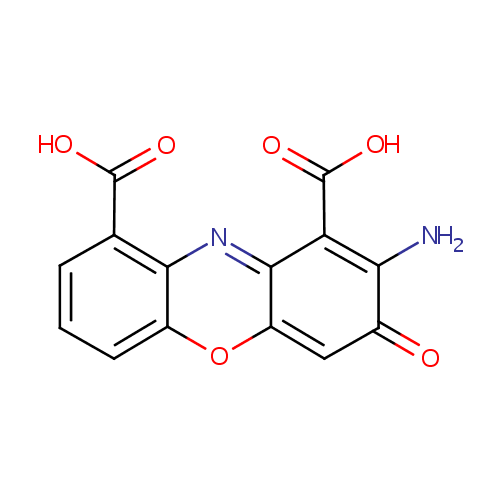
Structure |
|
|---|
| Synonyms: | - 2-Amino-3-oxo-3H-phenoxazin-1,9-dicarboxylate
- 2-Amino-3-oxo-3H-phenoxazin-1,9-dicarboxylic acid
- 2-Amino-3H-phenoxazin-one-1,9-dicarboxylate
- 2-Amino-3H-phenoxazin-one-1,9-dicarboxylic acid
- Cinnabarinate
- Cinnabarinic acid
- Cinnavalininic acid
|
|---|
|
Chemical Formula: |
C14H8N2O6 |
|---|
| Average Molecular Weight: |
300.2231 |
|---|
| Monoisotopic Molecular
Weight: |
300.038235998 |
|---|
| InChI Key: |
FSBKJYLVDRVPTK-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C14H8N2O6/c15-10-6(17)4-8-12(9(10)14(20)21)16-11-5(13(18)19)2-1-3-7(11)22-8/h1-4H,15H2,(H,18,19)(H,20,21) |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 2-amino-3-oxo-3H-phenoxazine-1,9-dicarboxylic acid |
|---|
|
Traditional IUPAC Name: |
cinnabarinic acid |
|---|
| SMILES: | NC1=C(C(O)=O)C2=NC3=C(C=CC=C3OC2=CC1=O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as phenoxazines. These are polycyclic aromatic compounds containing a phenoxazine moiety, which is a linear tricyclic system that consists of a two benzene rings joined by a 1,4-oxazine ring. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Benzoxazines |
|---|
| Sub Class | Phenoxazines |
|---|
|
Direct Parent |
Phenoxazines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Phenoxazine
- Aminobenzoic acid or derivatives
- Aminobenzoic acid
- Benzoyl
- Benzenoid
- Primary aromatic amine
- Dicarboxylic acid or derivatives
- Heteroaromatic compound
- Vinylogous amide
- Cyclic ketone
- Oxacycle
- Azacycle
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Amine
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -2 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | HMDB04078 | | Pubchem Compound ID | 114918 | | Kegg ID | C05640 | | ChemSpider ID | 102864 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|