Search Results for compounds
Searching compounds for
returned 4373 results.
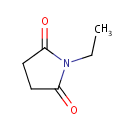
N-Ethylsuccinimide (PAMDB001329)
IUPAC:
1-ethylpyrrolidine-2,5-dione
CAS: 2314-78-5
Description: N-ethylsuccinimide is a member of the chemical class known as Pyrrolidones. These are compounds containing a pyrrolidine ring which bears a ketone. It is a product of the reduction of of N-ethylmaleimide (NEM) by N-ethylmaleimide reductase.
Heptosyl-KDO2-lipid A (PAMDB001334)
IUPAC:
(2R,4R,5R,6R)-4-{[6-(1,2-dihydroxyethyl)-2-(dihydroxymethyl)-4,5-dihydroxyoxan-2-yl]oxy}-6-[(1R)-1,2-dihydroxyethyl]-5-{[(3S,4S,5S)-6-[(1S)-1,2-dihydroxyethyl]-3,4,5-trihydroxyoxan-2-yl]oxy}-2-{[(2R,3S,4R,5R,6R)-6-{[(2R,3S,4R,5R,6R)-5-{[(3R)-1,3-dihydroxytetradecylidene]amino}-3-hydroxy-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonooxy)oxan-2-yl]methoxy}-5-{[(3R)-3-(dodecanoyloxy)-1-hydroxytetradecylidene]amino}-3-(phosphonooxy)-4-{[(3R)-3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl]methoxy}oxane-2-carboxylic acid
CAS: Not Available
Description: Heptosyl-kdo2-lipid a belongs to the class of Hexose Oligosaccharides. These are oligosaccharides in which the saccharide units are hexoses. (inferred from compound structure)
Heptosyl2-KDO2-lipid A (PAMDB001335)
IUPAC:
(2R,4R,5R,6R)-4-{[6-(1,2-dihydroxyethyl)-2-(dihydroxymethyl)-4,5-dihydroxyoxan-2-yl]oxy}-6-[(1R)-1,2-dihydroxyethyl]-5-{[(3S,4S,5R)-6-[(1S)-1,2-dihydroxyethyl]-4-{[(3S,4S,5S)-6-[(1S)-1,2-dihydroxyethyl]-3,4,5-trihydroxyoxan-2-yl]oxy}-3,5-dihydroxyoxan-2-yl]oxy}-2-{[(2R,3S,4R,5R,6R)-6-{[(2R,3S,4R,5R,6R)-5-{[(3R)-1,3-dihydroxytetradecylidene]amino}-3-hydroxy-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonooxy)oxan-2-yl]methoxy}-5-{[(3R)-3-(dodecanoyloxy)-1-hydroxytetradecylidene]amino}-3-(phosphonooxy)-4-{[(3R)-3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl]methoxy}oxane-2-carboxylic acid
CAS: Not Available
Description: Heptosyl2-kdo2-lipid A is a component of lipopolysaccharide. Bacterial lipopolysaccharides (LPS) typically consist of a hydrophobic domain inserted into the outer membrane known as lipid A (or endotoxin), a phosphorylated "core" oligosaccharide and a distal polysaccharide (or O antigen). The core oligosaccharides are conceptually divided into two regions: inner core and outer core. The inner core is highly conserved, comprises three deoxy-D-manno-octulosonic acid (KDO) and L-glycero-D-manno-heptose (Hep) and is often phosphorylated. The inner core oligosaccharide plays a critical role in essential barrier function of the outer membrane. The outer core comprises a tri-hexose backbone modified with varying side-branch substitutions of hexose and acetamidohexose residues. The outer core provides an attachment site for O-antigen. The completed lipid A-KDO2 serves as the acceptor on which the core oligosaccharide chains are assembled by sequential glycosyl transfer from nucleotide sugar precursors. This process involves a co-ordinated complex of membrane-associated glycosyltransferases acting at the cytoplasmic face of the plasma membrane.
Glucosyl-heptosyl2-KDO2-lipid A (PAMDB001336)
IUPAC:
(2R,4R,5R,6R)-4-{[6-(1,2-dihydroxyethyl)-2-(dihydroxymethyl)-4,5-dihydroxyoxan-2-yl]oxy}-6-[(1R)-1,2-dihydroxyethyl]-5-{[(3S,4S,5R)-6-[(1S)-1,2-dihydroxyethyl]-4-{[(3S,4S,5R)-6-[(1S)-1,2-dihydroxyethyl]-3,5-dihydroxy-4-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-2-yl]oxy}-3,5-dihydroxyoxan-2-yl]oxy}-2-{[(2R,3S,4R,5R,6R)-6-{[(2R,3S,4R,5R,6R)-5-{[(3R)-1,3-dihydroxytetradecylidene]amino}-3-hydroxy-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonooxy)oxan-2-yl]methoxy}-5-{[(3R)-3-(dodecanoyloxy)-1-hydroxytetradecylidene]amino}-3-(phosphonooxy)-4-{[(3R)-3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl]methoxy}oxane-2-carboxylic acid
CAS: Not Available
Description: Glucosyl-heptosyl2-kdo2-lipid a belongs to the class of Hexose Oligosaccharides. These are oligosaccharides in which the saccharide units are hexoses. (inferred from compound structure)
Glucosyl-heptosyl2-KDO2-lipid A-phosphate (PAMDB001337)
IUPAC:
{[(3R,4R,5S)-6-{[(2R,3R,4R,6R)-6-carboxy-4-{[6-(1,2-dihydroxyethyl)-2-(dihydroxymethyl)-4,5-dihydroxyoxan-2-yl]oxy}-2-[(1R)-1,2-dihydroxyethyl]-6-{[(2R,3S,4R,5R,6R)-6-{[(2R,3S,4R,5R,6R)-5-{[(3R)-1,3-dihydroxytetradecylidene]amino}-3-hydroxy-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonooxy)oxan-2-yl]methoxy}-5-{[(3R)-3-(dodecanoyloxy)-1-hydroxytetradecylidene]amino}-3-(phosphonooxy)-4-{[(3R)-3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl]methoxy}oxan-3-yl]oxy}-2-(1,2-dihydroxyethyl)-4-{[(3S,4S,5R)-6-[(1S)-1,2-dihydroxyethyl]-3,5-dihydroxy-4-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-2-yl]oxy}-5-hydroxyoxan-3-yl]oxy}trihydroxyphosphanium
CAS: Not Available
Description: Glucosyl-heptosyl2-kdo2-lipid A-phosphate is a component of LPS. Bacterial lipopolysaccharides (LPS) typically consist of a hydrophobic domain inserted into the outer membrane known as lipid A (or endotoxin), a phosphorylated "core" oligosaccharide and a distal polysaccharide (or O antigen). The core oligosaccharides are conceptually divided into two regions: inner core and outer core. The inner core is highly conserved, comprises three deoxy-D-manno-octulosonic acid (KDO) and L-glycero-D-manno-heptose (Hep) and is often phosphorylated. The inner core oligosaccharide plays a critical role in essential barrier function of the outer membrane. The outer core comprises a tri-hexose backbone modified with varying side-branch substitutions of hexose and acetamidohexose residues. The outer core provides an attachment site for O-antigen. The completed lipid A-KDO2 serves as the acceptor on which the core oligosaccharide chains are assembled by sequential glycosyl transfer from nucleotide sugar precursors. This process involves a co-ordinated complex of membrane-associated glycosyltransferases acting at the cytoplasmic face of the plasma membrane.
Lipid A-core (PAMDB001343)
IUPAC:
{[(3R,4R,5S)-6-{[(2R,3R,4R,6R)-6-carboxy-2-[(1R)-1,2-dihydroxyethyl]-4-{[(2R,4R,5R,6R)-6-[(1R)-1,2-dihydroxyethyl]-2-(dihydroxymethyl)-4,5-dihydroxyoxan-2-yl]oxy}-6-{[(2R,3S,4R,5R,6R)-6-{[(2R,3S,4R,5R,6R)-5-{[(3R)-1,3-dihydroxytetradecylidene]amino}-3-hydroxy-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonooxy)oxan-2-yl]methoxy}-5-{[(3R)-3-(dodecanoyloxy)-1-hydroxytetradecylidene]amino}-3-(phosphonooxy)-4-{[(3R)-3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl]methoxy}oxan-3-yl]oxy}-2-[(1S)-1,2-dihydroxyethyl]-4-{[(3S,4R,5R)-6-[(1S)-2-{[(3S,4S,5S)-6-[(1S)-1,2-dihydroxyethyl]-3,4,5-trihydroxyoxan-2-yl]oxy}-1-hydroxyethyl]-4-{[(3R,5R,6R)-4-{[(4S,5S,6R)-3-{[(3R,4S,5S,6R)-6-({[(3S,4S,5S)-6-[(1S)-1,2-dihydroxyethyl]-3,4,5-trihydroxyoxan-2-yl]oxy}methyl)-3,4,5-trihydroxyoxan-2-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,5-dihydroxy-6-({[(3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}methyl)oxan-2-yl]oxy}-3-hydroxy-5-[(trihydroxyphosphaniumyl)oxy]oxan-2-yl]oxy}-5-hydroxyoxan-3-yl]oxy}trihydroxyphosphanium
CAS: Not Available
Description: Lipid a-core belongs to the class of Polyhexoses. These are polysaccharides in which the saccharide units are hexoses. (inferred from compound structure) Lipopolysaccharide (LPS) is a major component on the surface of Gram negative bacteria and is composed of lipid A-core and the O antigen polysaccharide. (PMID 20333251) WaaL is a membrane enzyme implicated in ligating undecaprenyl-diphosphate (Und-PP)-linked O antigen to lipid A-core oligosaccharide. (PMID 19019161) MsbA is an essential ATP-binding cassette half-transporter in the cytoplasmic membrane of the gram-negative Pseudomonas aeruginosa and is required for the export of lipopolysaccharides (LPS) to the outer membrane, most likely by transporting the lipid A core moiety. (PMID 16159769)
TDP-Rhamnose (PAMDB001344)
IUPAC:
4-hydroxy-1-[(2S,4S,5S)-4-hydroxy-5-[2-({[(2R,3R,4R,5S,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl phosphonato]oxy}phosphinato)ethyl]oxolan-2-yl]-5-methyl-2-oxo-2,5-dihydro-1???pyrimidine-1,5-bis(ylium)
CAS: Not Available
Description: TDP-rhamnose is a member of the chemical class known as Mixed Pentose/Hexose Disaccharides. These are disaccharides containing both an hexose and a pentose. dTDP-rhamnose is an important precursor of cell wall polysaccharides and rhamnose-containing exopolysaccharides (EPS) in Pseudomonas aeruginosa. (PMID 14973085)
D-Myo-inositol (1)-monophosphate (PAMDB001350)
IUPAC:
{[(2R,3R,5S,6R)-2,3,4,5,6-pentahydroxycyclohexyl]oxy}phosphonic acid
CAS: 15421-51-9
Description: D-myo-inositol (1)-monophosphate is the D-isomer of myo-inositol 1-phosphate. It is a constituent of phospholipids and inositol polyphosphates. (EcoCyc) D-myo-inositol (1)-monophosphate can be converted to myo-inositol through the action of the enzyme myo-inositol-1(or 4)-monophosphatase (an inositol monophosphatase) (EC:3.1.3.25). (KEGG)
Dihydromonapterin-triphosphate (PAMDB001355)
IUPAC:
6-[(1S,2S)-3-[({[(hydrogen phosphonatooxy)phosphinato]oxy}phosphinato)-??-oxidanyliumyl]-1,2-dihydroxypropyl]-4-hydroxy-2-iminiumyl-2,7-dihydro-1H-5???pteridin-8-ium-5-ylium
CAS: Not Available
Description: Dihydromonapterin-triphosphate is a member of the chemical class known as Biopterins and Derivatives. These are coenzymes containing a 2-amino-pteridine-4-one derivative. 7,8-Dihydroneopterin triphosphate (DHNTP) is an intermediate in tetrahydrobiopterin and tetrahydromonopterin biosynthesis. Tetrahydromonapterin is the major tetrahydropterin in Pseudomonas aeruginosa, although the biological role of tetrahydromonapterin in Pseudomonas aeruginosa is currently unknown.
Heme D (PAMDB001368)
IUPAC:
3-[20-(2-carboxyethyl)-10,15-bis(carboxymethyl)-5,10,15,19-tetramethyl-9,14-dioxo-21,22,23,24-tetraazapentacyclo[16.2.1.1?,??1????.1??,???tetracosa-1(20),2,4,6,8(23),11,13(22),16,18-nonaen-4-yl]propanoic acid
CAS: 60318-31-2
Description: Heme D is a member of the chemical class known as tetrapyrroles and Derivatives. These are polycyclic aromatic compounds containing four pyrrole rings joined by one-carbon units linking position 2 of one pyrrole ring to position 5 of the next. (inferred from compound structure). A heme (American English) or haem (British English) is a prosthetic group that consists of an iron atom contained in the center of a large heterocyclic organic ring called a porphyrin. Heme D is a derivative of heme B, but in which the propionic acid side chain at the carbon of position 6, which is also hydroxylated, forms a ??-spirolactone. Ring III is also hydroxylated at position 5, in a conformation trans to the new lactone group. Heme D is the site for oxygen reduction to water of many types of bacteria at low oxygen tension. In Pseudomonas aeruginosa, heme D is formed from protoheme in the interior of the catalase hydroperoxidase II (HPII) molecule through a self-catalyzed reaction. [PMID: 8621527]