|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001335 |
|---|
|
Identification |
|---|
| Name: |
Heptosyl2-KDO2-lipid A |
|---|
| Description: | Heptosyl2-kdo2-lipid A is a component of lipopolysaccharide. Bacterial lipopolysaccharides (LPS) typically consist of a hydrophobic domain inserted into the outer membrane known as lipid A (or endotoxin), a phosphorylated "core" oligosaccharide and a distal polysaccharide (or O antigen). The core oligosaccharides are conceptually divided into two regions: inner core and outer core. The inner core is highly conserved, comprises three deoxy-D-manno-octulosonic acid (KDO) and L-glycero-D-manno-heptose (Hep) and is often phosphorylated. The inner core oligosaccharide plays a critical role in essential barrier function of the outer membrane. The outer core comprises a tri-hexose backbone modified with varying side-branch substitutions of hexose and acetamidohexose residues. The outer core provides an attachment site for O-antigen. The completed lipid A-KDO2 serves as the acceptor on which the core oligosaccharide chains are assembled by sequential glycosyl transfer from nucleotide sugar precursors. This process involves a co-ordinated complex of membrane-associated glycosyltransferases acting at the cytoplasmic face of the plasma membrane. |
|---|
|
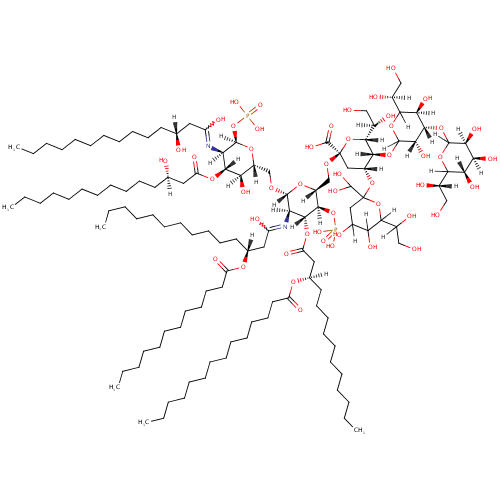
Structure |
|
|---|
| Synonyms: | - (heptosyl)2-(KDO)2-lipid A
- Heptosyl-heptosyl-kdo2-lipidA
- Heptosyl2-KDO2-lipid A
|
|---|
|
Chemical Formula: |
C124H228N2O51P2 |
|---|
| Average Molecular Weight: |
2625.0674 |
|---|
| Monoisotopic Molecular
Weight: |
2623.478424052 |
|---|
| InChI Key: |
MVLFKXQHQYOOOH-JMRRDJIDSA-N |
|---|
| InChI: | InChI=1S/C124H228N2O51P2/c1-7-13-19-25-31-37-38-44-50-56-62-68-96(141)164-84(66-60-54-48-42-35-29-23-17-11-5)72-98(143)168-115-100(126-94(139)71-83(65-59-53-47-41-34-28-22-16-10-4)163-95(140)67-61-55-49-43-36-30-24-18-12-6)117(161-79-91-102(145)114(167-97(142)70-82(132)64-58-52-46-40-33-27-21-15-9-3)99(118(165-91)177-179(158,159)160)125-93(138)69-81(131)63-57-51-45-39-32-26-20-14-8-2)166-92(113(115)176-178(155,156)157)80-162-123(121(151)152)74-90(173-124(122(153)154)73-85(133)101(144)110(174-124)88(136)77-129)112(111(175-123)89(137)78-130)171-120-107(150)116(106(149)109(170-120)87(135)76-128)172-119-105(148)103(146)104(147)108(169-119)86(134)75-127/h81-92,99-120,122,127-137,144-150,153-154H,7-80H2,1-6H3,(H,125,138)(H,126,139)(H,151,152)(H2,155,156,157)(H2,158,159,160)/t81-,82-,83-,84-,85?,86+,87+,88?,89-,90-,91-,92-,99-,100-,101?,102-,103+,104+,105+,106-,107+,108?,109?,110?,111-,112-,113-,114-,115-,116+,117-,118-,119?,120?,123-,124?/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (2R,4R,5R,6R)-4-{[6-(1,2-dihydroxyethyl)-2-(dihydroxymethyl)-4,5-dihydroxyoxan-2-yl]oxy}-6-[(1R)-1,2-dihydroxyethyl]-5-{[(3S,4S,5R)-6-[(1S)-1,2-dihydroxyethyl]-4-{[(3S,4S,5S)-6-[(1S)-1,2-dihydroxyethyl]-3,4,5-trihydroxyoxan-2-yl]oxy}-3,5-dihydroxyoxan-2-yl]oxy}-2-{[(2R,3S,4R,5R,6R)-6-{[(2R,3S,4R,5R,6R)-5-{[(3R)-1,3-dihydroxytetradecylidene]amino}-3-hydroxy-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonooxy)oxan-2-yl]methoxy}-5-{[(3R)-3-(dodecanoyloxy)-1-hydroxytetradecylidene]amino}-3-(phosphonooxy)-4-{[(3R)-3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl]methoxy}oxane-2-carboxylic acid |
|---|
|
Traditional IUPAC Name: |
(2R,4R,5R,6R)-4-{[6-(1,2-dihydroxyethyl)-2-(dihydroxymethyl)-4,5-dihydroxyoxan-2-yl]oxy}-6-[(1R)-1,2-dihydroxyethyl]-5-{[(3S,4S,5R)-6-[(1S)-1,2-dihydroxyethyl]-4-{[(3S,4S,5S)-6-[(1S)-1,2-dihydroxyethyl]-3,4,5-trihydroxyoxan-2-yl]oxy}-3,5-dihydroxyoxan-2-yl]oxy}-2-{[(2R,3S,4R,5R,6R)-6-{[(2R,3S,4R,5R,6R)-5-{[(3R)-1,3-dihydroxytetradecylidene]amino}-3-hydroxy-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonooxy)oxan-2-yl]methoxy}-5-{[(3R)-3-(dodecanoyloxy)-1-hydroxytetradecylidene]amino}-3-(phosphonooxy)-4-{[(3R)-3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl]methoxy}oxane-2-carboxylic acid |
|---|
| SMILES: | [H][C@@](O)(CCCCCCCCCCC)CC(=O)O[C@@]1([H])[C@]([H])(O)[C@@]([H])(CO[C@]2([H])O[C@]([H])(CO[C@@]3(C[C@@]([H])(OC4(CC([H])(O)C([H])(O)C([H])(O4)C([H])(O)CO)C(O)O)[C@@]([H])(OC4([H])OC([H])([C@@]([H])(O)CO)[C@@]([H])(O)[C@]([H])(OC5([H])OC([H])([C@@]([H])(O)CO)[C@@]([H])(O)[C@]([H])(O)[C@]5([H])O)[C@]4([H])O)[C@]([H])(O3)[C@]([H])(O)CO)C(O)=O)[C@@]([H])(OP(O)(O)=O)[C@]([H])(OC(=O)C[C@@]([H])(CCCCCCCCCCC)OC(=O)CCCCCCCCCCCCC)[C@@]2([H])N=C(O)C[C@@]([H])(CCCCCCCCCCC)OC(=O)CCCCCCCCCCC)O[C@]([H])(OP(O)(O)=O)[C@]1([H])N=C(O)C[C@]([H])(O)CCCCCCCCCCC |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as n-acyl-alpha-hexosamines. These are carbohydrate derivatives containing a hexose moiety in which the oxygen atom is replaced by an n-acyl group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organooxygen compounds |
|---|
|
Class |
Carbohydrates and carbohydrate conjugates |
|---|
| Sub Class | Aminosaccharides |
|---|
|
Direct Parent |
N-acyl-alpha-hexosamines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Oligosaccharide phosphate
- Oligosaccharide
- Saccharolipid
- Fatty acyl glycoside
- Pentacarboxylic acid or derivatives
- N-acyl-alpha-hexosamine
- C-glucuronide
- Glucosamine
- Alkyl glycoside
- O-glycosyl compound
- Glycosyl compound
- C-glycosyl compound
- Pyran carboxylic acid
- Pyran carboxylic acid or derivatives
- Monoalkyl phosphate
- Fatty acid ester
- Beta-hydroxy acid
- Fatty acyl
- Alkyl phosphate
- Pyran
- Phosphoric acid ester
- Oxane
- Organic phosphoric acid derivative
- Organic phosphate
- Hydroxy acid
- Secondary alcohol
- Polyol
- Carboxylic acid ester
- 1,2-diol
- Oxacycle
- Organoheterocyclic compound
- 1,1-diol
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboxylic acid
- Carboxylic acid derivative
- Carboximidic acid derivative
- Carboximidic acid
- Carbonyl hydrate
- Acetal
- Hydrocarbon derivative
- Primary alcohol
- Organonitrogen compound
- Carbonyl group
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Membrane |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Lipopolysaccharide biosynthesis pae00540
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | 25202915 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | CPD0-930 | | EcoCyc ID | CPD0-930 |
|
|---|