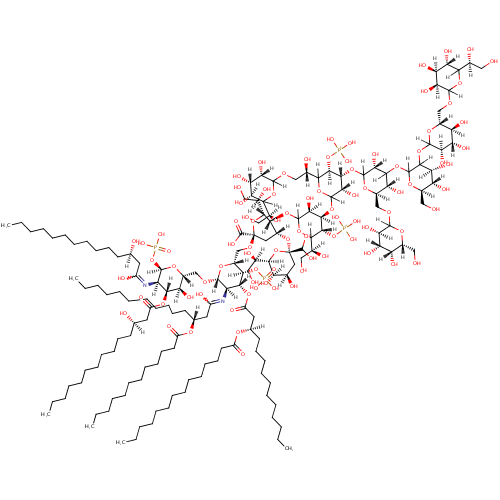
Lipid A-core (PAMDB001343)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB001343 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Lipid A-core | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Lipid a-core belongs to the class of Polyhexoses. These are polysaccharides in which the saccharide units are hexoses. (inferred from compound structure) Lipopolysaccharide (LPS) is a major component on the surface of Gram negative bacteria and is composed of lipid A-core and the O antigen polysaccharide. (PMID 20333251) WaaL is a membrane enzyme implicated in ligating undecaprenyl-diphosphate (Und-PP)-linked O antigen to lipid A-core oligosaccharide. (PMID 19019161) MsbA is an essential ATP-binding cassette half-transporter in the cytoplasmic membrane of the gram-negative Pseudomonas aeruginosa and is required for the export of lipopolysaccharides (LPS) to the outer membrane, most likely by transporting the lipid A core moiety. (PMID 16159769) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C162H296N2O89P4 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 3819.9387 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 3817.764804888 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | PIKXVLIJAUWPDI-VUQADABISA-P | |||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C162H294N2O89P4/c1-7-13-19-25-31-37-38-44-50-56-62-68-108(184)229-90(66-60-54-48-42-35-29-23-17-11-5)72-110(186)237-142-112(164-106(182)71-89(65-59-53-47-41-34-28-22-16-10-4)228-107(183)67-61-55-49-43-36-30-24-18-12-6)149(224-84-102-117(191)141(236-109(185)70-88(173)64-58-52-46-40-33-27-21-15-9-3)111(150(232-102)253-257(220,221)222)163-105(181)69-87(172)63-57-51-45-39-32-26-20-14-8-2)235-104(140(142)250-254(211,212)213)86-227-161(159(207)208)74-98(247-162(160(209)210)73-91(174)113(187)135(248-162)94(177)77-167)139(138(249-161)96(179)79-169)242-156-131(205)144(147(251-255(214,215)216)136(240-156)95(178)78-168)245-157-132(206)145(148(252-256(217,218)219)137(241-157)97(180)82-223-152-127(201)121(195)124(198)133(238-152)92(175)75-165)244-155-130(204)143(118(192)103(234-155)85-225-151-126(200)119(193)114(188)99(80-170)230-151)243-158-146(123(197)115(189)100(81-171)231-158)246-154-129(203)120(194)116(190)101(233-154)83-226-153-128(202)122(196)125(199)134(239-153)93(176)76-166/h87-104,111-158,160,165-180,187-206,209-210,214-219H,7-86H2,1-6H3,(H5-2,163,164,181,182,207,208,211,212,213,220,221,222)/p+2/t87-,88-,89-,90-,91-,92+,93+,94-,95+,96-,97+,98-,99-,100-,101-,102-,103-,104-,111-,112-,113-,114+,115-,116-,117-,118-,119+,120+,121+,122+,123+,124+,125+,126-,127+,128+,129-,130-,131+,132+,133?,134?,135-,136?,137?,138-,139-,140-,141-,142-,143?,144-,145-,146?,147-,148-,149-,150-,151?,152?,153?,154?,155?,156?,157?,158?,161-,162-/m1/s1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[(3R,4R,5S)-6-{[(2R,3R,4R,6R)-6-carboxy-2-[(1R)-1,2-dihydroxyethyl]-4-{[(2R,4R,5R,6R)-6-[(1R)-1,2-dihydroxyethyl]-2-(dihydroxymethyl)-4,5-dihydroxyoxan-2-yl]oxy}-6-{[(2R,3S,4R,5R,6R)-6-{[(2R,3S,4R,5R,6R)-5-{[(3R)-1,3-dihydroxytetradecylidene]amino}-3-hydroxy-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonooxy)oxan-2-yl]methoxy}-5-{[(3R)-3-(dodecanoyloxy)-1-hydroxytetradecylidene]amino}-3-(phosphonooxy)-4-{[(3R)-3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl]methoxy}oxan-3-yl]oxy}-2-[(1S)-1,2-dihydroxyethyl]-4-{[(3S,4R,5R)-6-[(1S)-2-{[(3S,4S,5S)-6-[(1S)-1,2-dihydroxyethyl]-3,4,5-trihydroxyoxan-2-yl]oxy}-1-hydroxyethyl]-4-{[(3R,5R,6R)-4-{[(4S,5S,6R)-3-{[(3R,4S,5S,6R)-6-({[(3S,4S,5S)-6-[(1S)-1,2-dihydroxyethyl]-3,4,5-trihydroxyoxan-2-yl]oxy}methyl)-3,4,5-trihydroxyoxan-2-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,5-dihydroxy-6-({[(3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}methyl)oxan-2-yl]oxy}-3-hydroxy-5-[(trihydroxyphosphaniumyl)oxy]oxan-2-yl]oxy}-5-hydroxyoxan-3-yl]oxy}trihydroxyphosphanium | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | {[(3R,4R,5S)-6-{[(2R,3R,4R,6R)-6-carboxy-2-[(1R)-1,2-dihydroxyethyl]-4-{[(2R,4R,5R,6R)-6-[(1R)-1,2-dihydroxyethyl]-2-(dihydroxymethyl)-4,5-dihydroxyoxan-2-yl]oxy}-6-{[(2R,3S,4R,5R,6R)-6-{[(2R,3S,4R,5R,6R)-5-{[(3R)-1,3-dihydroxytetradecylidene]amino}-3-hydroxy-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonooxy)oxan-2-yl]methoxy}-5-{[(3R)-3-(dodecanoyloxy)-1-hydroxytetradecylidene]amino}-3-(phosphonooxy)-4-{[(3R)-3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl]methoxy}oxan-3-yl]oxy}-2-[(1S)-1,2-dihydroxyethyl]-4-{[(3S,4R,5R)-6-[(1S)-2-{[(3S,4S,5S)-6-[(1S)-1,2-dihydroxyethyl]-3,4,5-trihydroxyoxan-2-yl]oxy}-1-hydroxyethyl]-4-{[(3R,5R,6R)-4-{[(4S,5S,6R)-3-{[(3R,4S,5S,6R)-6-({[(3S,4S,5S)-6-[(1S)-1,2-dihydroxyethyl]-3,4,5-trihydroxyoxan-2-yl]oxy}methyl)-3,4,5-trihydroxyoxan-2-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3,5-dihydroxy-6-({[(3R,4S,5R,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}methyl)oxan-2-yl]oxy}-3-hydroxy-5-[(trihydroxyphosphaniumyl)oxy]oxan-2-yl]oxy}-5-hydroxyoxan-3-yl]oxy}trihydroxyphosphanium | |||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | [H][C@@](O)(CCCCCCCCCCC)CC(=O)O[C@@]1([H])[C@]([H])(O)[C@@]([H])(CO[C@]2([H])O[C@]([H])(CO[C@@]3(C[C@@]([H])(O[C@@]4(C[C@@]([H])(O)[C@@]([H])(O)[C@]([H])(O4)[C@]([H])(O)CO)C(O)O)[C@@]([H])(OC4([H])OC([H])([C@@]([H])(O)CO)[C@@]([H])(O[P+](O)(O)O)[C@]([H])(OC5([H])OC([H])([C@@]([H])(O)COC6([H])OC([H])([C@@]([H])(O)CO)[C@@]([H])(O)[C@]([H])(O)[C@]6([H])O)[C@@]([H])(O[P+](O)(O)O)[C@]([H])(OC6([H])O[C@]([H])(COC7([H])O[C@]([H])(CO)[C@]([H])(O)[C@]([H])(O)[C@@]7([H])O)[C@@]([H])(O)C([H])(OC7([H])O[C@]([H])(CO)[C@@]([H])(O)[C@]([H])(O)C7([H])OC7([H])O[C@]([H])(COC8([H])OC([H])([C@@]([H])(O)CO)[C@@]([H])(O)[C@]([H])(O)[C@]8([H])O)[C@@]([H])(O)[C@]([H])(O)[C@@]7([H])O)[C@@]6([H])O)[C@]5([H])O)[C@]4([H])O)[C@]([H])(O3)[C@]([H])(O)CO)C(O)=O)[C@@]([H])(OP(O)(O)=O)[C@]([H])(OC(=O)C[C@@]([H])(CCCCCCCCCCC)OC(=O)CCCCCCCCCCCCC)[C@@]2([H])N=C(O)C[C@@]([H])(CCCCCCCCCCC)OC(=O)CCCCCCCCCCC)O[C@]([H])(OP(O)(O)=O)[C@]1([H])N=C(O)C[C@]([H])(O)CCCCCCCCCCC | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as polysaccharides. These are compounds containing more than ten saccharide units. | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic Polymers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Polysaccharides | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Polysaccharides | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic heteromonocyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Membrane | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Lipid A-core + colanic acid > M<sub>LPS</sub> Galactosyl-glucosyl3-heptosyl3-KDO2-lipid A-bisphosphate + ADP-L-Glycero-D-manno-heptose > Hydrogen ion + Lipid A-core + ADP galactosyl-(glucosyl)3-(heptosyl)3-Kdo2-lipid A-bisphosphate + ADP-L-glycero-beta-D-manno-heptose > Hydrogen ion + Adenosine diphosphate + Lipid A-core + ADP Lipid A-core + Adenosine triphosphate + Water > Adenosine diphosphate + Phosphate + Hydrogen ion + Lipid A-core + ADP Lipid A-core + Adenosine triphosphate + Water > Adenosine diphosphate + Phosphate + Hydrogen ion + Lipid A-core + ADP | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in nucleotide binding
- Specific function:
- Involved in lipid A export and possibly also in glycerophospholipid export and for biogenesis of the outer membrane. Transmembrane domains (TMD) form a pore in the inner membrane and the ATP-binding domain (NBD) is responsible for energy generation
- Gene Name:
- msbA
- Locus Tag:
- PA4997
- Molecular weight:
- 66.4 kDa
Transporters
- General function:
- Involved in nucleotide binding
- Specific function:
- Involved in lipid A export and possibly also in glycerophospholipid export and for biogenesis of the outer membrane. Transmembrane domains (TMD) form a pore in the inner membrane and the ATP-binding domain (NBD) is responsible for energy generation
- Gene Name:
- msbA
- Locus Tag:
- PA4997
- Molecular weight:
- 66.4 kDa