Search Results for compounds
Searching compounds for
returned 4373 results.
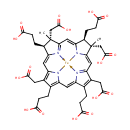
Siroheme (PAMDB001040)
IUPAC:
3-[(4S,5S,9S,10S,11Z,16Z)-9,15,19-tris(2-carboxyethyl)-5,10,14,20-tetrakis(carboxymethyl)-5,10-dimethyl-21,23,24,25-tetraaza-22-ferrahexacyclo[9.9.3.1?,??1??,???0????.0?????]pentacosa-1(20),2,6(25),7,11,13(24),14,16,18-nonaen-4-yl]propanoic acid
CAS: 52553-42-1
Description: Siroheme belongs to the class of Precorrins. These are intermediates formed by methylation at one or more of the four rings prior to the formation of the macrocyclic corrin ring. (inferred from compound structure)Siroheme (or sirohaem) is a heme-like prosthetic group used by some enzymes to accomplish the six-electron reduction of sulfur and nitrogen. Siroheme is synthesized from uroporphyrinogen III, a heme and vitamin B12 precursor. It plays a major role in the sulfur assimilation pathway: converting sulfite to a biologically useful sulfide, which can be incorporated into the organic compound homocysteine. (WikiPedia)
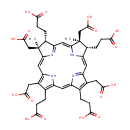
Sirohydrochlorin (PAMDB001041)
IUPAC:
3-[(4S,5S,9S,10S)-9,15,19-tris(2-carboxyethyl)-5,10,14,20-tetrakis(carboxymethyl)-5,10-dimethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1?,??1????.1??,???tetracosa-1(21),2,6,8(23),11,13,15,17,19-nonaen-4-yl]propanoic acid
CAS: 65207-12-7
Description: Sirohydrochlorin is a member of the chemical class known as Precorrins. These are intermediates formed by methylation at one or more of the four rings prior to the formation of the macrocyclic corrin ring. typhimurium, precorrin-2 is a precursor of both siroheme and B12. (PMID 8955319)
UDP-2,3-Bis(3-hydroxytetradecanoyl)glucosamine (PAMDB001047)
IUPAC:
(3R)-N-[(2R,3R,4R,5S,6R)-2-({[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(4-hydroxy-2-oxo-1,2-dihydropyrimidin-1-yl)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-5-hydroxy-6-(hydroxymethyl)-4-{[(3R)-3-hydroxytetradecanoyl]oxy}oxan-3-yl]-3-hydroxytetradecanimidic acid
CAS: Not Available
Description: UDP-2,3-bis(3-hydroxytetradecanoyl)glucosamine is a member of the chemical class known as Sulfanylbenzoic Acid Derivatives. These are benzoic acid derivatives which bear a sulfanyl group (R-SH) attached to the benzene ring.
UDP-N-Acetylmuramoyl-L-alanyl-D-gamma-glutamyl-meso-2,6-diaminopimelate (PAMDB001049)
IUPAC:
2-amino-6-{[(4R)-4-carboxy-4-{[(2S)-2-[(2-{[(2R,3R,4R,5S,6R)-2-({[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(4-hydroxy-2-oxo-1,2-dihydropyrimidin-1-yl)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-5-hydroxy-3-[(1-hydroxyethylidene)amino]-6-(hydroxymethyl)oxan-4-yl]oxy}-1-hydroxypropylidene)amino]-1-hydroxypropylidene]amino}-1-hydroxybutylidene]amino}heptanedioic acid
CAS: Not Available
Description: UDP-N-acetylmuramoyl-L-alanyl-D-gamma-glutamyl-meso-2,6-diaminopimelate is a peptide. It is a key component of peptidoglycan synthesis. The peptidoglycan synthesis pathway starts at the cytoplasm, where in six steps the peptidoglycan precursor a UDP-N-acetylmuramoyl-pentapeptide is synthesized. This precursor is then attached to the memberane acceptor all-trans-undecaprenyl phosphate, generating a N-acetylmuramoyl-pentapeptide-diphosphoundecaprenol, also known as lipid I. Another transferase then adds UDP-N-acetyl-D-glucosamine, yielding the complete monomeric unit a lipid II, also known as lipid II. This final lipid intermediate is transferred through the membrane. The peptidoglycan monomers are then polymerized on the outside surface by glycosyltransferases, which form the linear glycan chains, and transpeptidases, which catalyze the formation of peptide crosslinks.
UDP-N-Acetylmuramoyl-L-alanyl-D-glutamyl-L-lysyl-D-alanyl-D-alanine (PAMDB001051)
IUPAC:
4-{[(1S)-5-amino-1-{[(1R)-1-{[(1R)-1-carboxyethyl]-C-hydroxycarbonimidoyl}ethyl]-C-hydroxycarbonimidoyl}pentyl]-C-hydroxycarbonimidoyl}-2-{[(2S)-2-[(2-{[(3R,4R,5S,6R)-2-({[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(4-hydroxy-2-oxo-1,2-dihydropyrimidin-1-yl)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-5-hydroxy-3-[(1-hydroxyethylidene)amino]-6-(hydroxymethyl)oxan-4-yl]oxy}-1-hydroxypropylidene)amino]-1-hydroxypropylidene]amino}butanoic acid
CAS: Not Available
Description: UDP-N-acetylmuramoyl-L-alanyl-D-glutamyl-L-lysyl-D-alanyl-D-alanine is a member of the chemical class known as Peptides. These are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another. UDP-MurNAc-pentapeptide is a peptidoglycan precursor. It is a key component of peptidoglycan synthesis. The peptidoglycan synthesis pathway starts at the cytoplasm, where in six steps the peptidoglycan precursor a UDP-N-acetylmuramoyl-pentapeptide is synthesized. This precursor is then attached to the memberane acceptor all-trans-undecaprenyl phosphate, generating a N-acetylmuramoyl-pentapeptide-diphosphoundecaprenol, also known as lipid I. Another transferase then adds UDP-N-acetyl-alpha-D-glucosamine, yielding the complete monomeric unit a lipid , also known as lipid . This final lipid intermediate is transferred through the membrane. The peptidoglycan monomers are then polymerized on the outside surface by glycosyltransferases, which form the linear glycan chains, and transpeptidases, which catalyze the formation of peptide crosslinks. UDP-MurNAc-pentapeptide is a feedback inhibitor of UDP-MurNAc-peptide synthesis. (PMID 357424)
UDP-N-Acetylmuramoyl-L-alanyl-gamma-D-glutamyl-L-lysine (PAMDB001052)
IUPAC:
(2S)-6-amino-2-{[(4R)-4-carboxy-4-{[(2S)-2-{[(2R)-2-{[(2R,3R,4R,5S,6R)-2-({[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(4-hydroxy-2-oxo-1,2-dihydropyrimidin-1-yl)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-5-hydroxy-3-[(1-hydroxyethylidene)amino]-6-(hydroxymethyl)oxan-4-yl]oxy}-1-hydroxypropylidene]amino}-1-hydroxypropylidene]amino}-1-hydroxybutylidene]amino}hexanoic acid
CAS: Not Available
Description: UDP-N-acetylmuramoyl-L-alanyl-gamma-D-glutamyl-L-lysine is a member of the chemical class known as Peptides. These are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another. It is a key component of peptidoglycan synthesis. The peptidoglycan synthesis pathway starts at the cytoplasm, where in six steps the peptidoglycan precursor a UDP-N-acetylmuramoyl-pentapeptide is synthesized. This precursor is then attached to the memberane acceptor all-trans-undecaprenyl phosphate, generating a N-acetylmuramoyl-pentapeptide-diphosphoundecaprenol, also known as lipid I. Another transferase then adds UDP-N-acetyl-alpha-D-glucosamine, yielding the complete monomeric unit a lipid , also known as lipid . This final lipid intermediate is transferred through the membrane. The peptidoglycan monomers are then polymerized on the outside surface by glycosyltransferases, which form the linear glycan chains, and transpeptidases, which catalyze the formation of peptide crosslinks.
Undecaprenyl phosphate (PAMDB001054)
IUPAC:
{[(2E,6E,10E,14E,18E,22E,26E,30E,34E,38E)-3,7,11,15,19,23,27,31,35,39,43-undecamethyltetratetraconta-2,6,10,14,18,22,26,30,34,38,42-undecaen-1-yl]oxy}phosphonic acid
CAS: 25126-51-6
Description: Undecaprenyl phosphate belongs to the class of Polyprenyl Phosphates. These are prenol lipids in which the phosphate group is linked to one end of the polyprenol moiety. (inferred from compound structure)C55-isoprenyl pyrophosphate (undecaprenyl pyrophosphate) is an essential molecule involved in construction of the bacterial peptidoglycan cell wall. (WikiPedia)
Undecaprenyl-diphospho-N-acetylmuramoyl-(N-acetylglucosamine)-L-alanyl-D-glutaminyl-meso-2,6-diaminopimeloyl-D-alanyl-D-alanine (PAMDB001056)
IUPAC:
(2S,6R)-2-amino-6-{[(1R)-1-{[(1R)-1-carboxyethyl]-C-hydroxycarbonimidoyl}ethyl]-C-hydroxycarbonimidoyl}-6-{[(2R)-2-{[(2S)-2-[(2-{[(2R,3S,4R,5R)-3-{[(2S,3R,4R,5S,6R)-4,5-dihydroxy-3-[(1-hydroxyethylidene)amino]-6-(hydroxymethyl)oxan-2-yl]oxy}-6-{[hydroxy({[hydroxy({[(2E,6E,10E,14E,18E,22E,26E,30E,34E,38E)-3,7,11,15,19,23,27,31,35,39,43-undecamethyltetratetraconta-2,6,10,14,18,22,26,30,34,38,42-undecaen-1-yl]oxy})phosphoryl]oxy})phosphoryl]oxy}-5-[(1-hydroxyethylidene)amino]-2-(hydroxymethyl)oxan-4-yl]oxy}-1-hydroxypropylidene)amino]-1-hydroxypropylidene]amino}-1-hydroxy-4-(C-hydroxycarbonimidoyl)butylidene]amino}hexanoic acid
CAS: Not Available
Description: Undecaprenyl-diphospho-n-acetylmuramoyl-(n-acetylglucosamine)-l-alanyl-d-glutaminyl-meso-2,6-diaminopimeloyl-d-alanyl-d-alanine is a peptide. It is a key component of peptidoglycan synthesis. The peptidoglycan synthesis pathway starts at the cytoplasm, where in six steps the peptidoglycan precursor a UDP-N-acetylmuramoyl-pentapeptide is synthesized. This precursor is then attached to the memberane acceptor all-trans-undecaprenyl phosphate, generating a N-acetylmuramoyl-pentapeptide-diphosphoundecaprenol, also known as lipid I. Another transferase then adds UDP-N-acetyl-D-glucosamine, yielding the complete monomeric unit a lipid II, also known as lipid II. This final lipid intermediate is transferred through the membrane. The peptidoglycan monomers are then polymerized on the outside surface by glycosyltransferases, which form the linear glycan chains, and transpeptidases, which catalyze the formation of peptide crosslinks.
Undecaprenyl-diphospho-N-acetylmuramoyl-(N-acetylglucosamine)-L-alanyl-D-glutamyl-meso-2,6-diaminopimeloyl-D-alanyl-D-alanine (PAMDB001058)
IUPAC:
(2S,6R)-2-amino-6-{[(2R)-4-carboxy-2-{[(2S)-2-[(2-{[(2R,3S,4R,5R,6R)-3-{[(2S,3R,4R,5S,6R)-4,5-dihydroxy-3-[(1-hydroxyethylidene)amino]-6-(hydroxymethyl)oxan-2-yl]oxy}-6-{[hydroxy({[hydroxy({[(2E,6E,10E,14E,18E,22E,26E,30E,34E,38E)-3,7,11,15,19,23,27,31,35,39,43-undecamethyltetratetraconta-2,6,10,14,18,22,26,30,34,38,42-undecaen-1-yl]oxy})phosphoryl]oxy})phosphoryl]oxy}-5-[(1-hydroxyethylidene)amino]-2-(hydroxymethyl)oxan-4-yl]oxy}-1-hydroxypropylidene)amino]-1-hydroxypropylidene]amino}-1-hydroxybutylidene]amino}-6-{[(1R)-1-{[(1R)-1-carboxyethyl]-C-hydroxycarbonimidoyl}ethyl]-C-hydroxycarbonimidoyl}hexanoic acid
CAS: Not Available
Description: Undecaprenyl-diphospho-N-acetylmuramoyl-(N-acetylglucosamine)-L-alanyl-D-glutamyl-meso-2,6-diaminopimeloyl-D-alanyl-D-alanine is an intermediate in peptidoglycan synthesis. It is a substrate for the enzyme undecaprenyldiphospho-muramoylpentapeptide beta-N-acetylglucosaminyltransferase (murG). Peptidoglycan is best described as a fisherman net. The mesh of the net is made of two segments of parallel, rather inextensible glycan threads, held together by two small elastic peptide crosslinks allowing the net to expand or shrink. The glycan moiety of the peptidoglycan is remarkably uniform among all bacteria, and is made up of alternating β-1,4-linked N-acetylglucosamine and N-acetyl muramate residues, with an average chain length (in different organisms) of 10 to 65 disaccharide units. The peptidoglycan synthesis pathway starts at the cytoplasm, where in six steps the peptidoglycan precursor a UDP-N-acetylmuramoyl-pentapeptide is synthesized. This precursor is then attached to the memberane acceptor all-trans-undecaprenyl phosphate, generating a N-acetylmuramoyl-pentapeptide-diphosphoundecaprenol, also known as lipid I. Another transferase then adds UDP-N-acetyl-α-D-glucosamine, yielding the complete monomeric unit a lipid II, also known as lipid II. This final lipid intermediate is transferred by an as yet unknown mechanism through the membrane. The peptidoglycan monomers are then polymerized on the outside surface by glycosyltransferases, which form the linear glycan chains, and transpeptidases, which catalyze the formation of peptide crosslinks. Peptide crosslinks form between different parts of the peptides depending on the organism. For example, in Mycobacteria and in Pseudomonas aeruginosa most links form between the carboxyl group of the penultimate D-alanine (residue 4) of one peptide to the amino group at the D-center of meso-diaminopimelate (residue 3) of an adjacent peptide of a second glycan chain (as in Pseudomonas aeruginosa). The crosslinking reaction is catalyzed by transpeptidases and involves the cleavage of the D-alanyl-D-alanine bond of the donor peptide, providing the energy to drive the reaction. As a result, the peptides in the peptidoglycan polymers are one or two amino acids shorter than the peptides in the monomers.
Undecaprenyl-diphospho-N-acetylmuramoyl-(N-acetylglucosamine)-L-alanyl-gamma-D-glutamyl-L-lysyl-D-alanyl-D-alanine (PAMDB001060)
IUPAC:
4-{[(1S)-5-amino-1-{[(1R)-1-{[(1R)-1-carboxyethyl]-C-hydroxycarbonimidoyl}ethyl]-C-hydroxycarbonimidoyl}pentyl]-C-hydroxycarbonimidoyl}-2-{[(2S)-2-[(2-{[(2R,3S,4R,5R,6R)-3-{[(2S,3R,4R,5S,6R)-4,5-dihydroxy-3-[(1-hydroxyethylidene)amino]-6-(hydroxymethyl)oxan-2-yl]oxy}-6-{[hydroxy({[hydroxy({[(2E,6E,10E,14E,18E,22E,26E,30E,34E,38E)-3,7,11,15,19,23,27,31,35,39,43-undecamethyltetratetraconta-2,6,10,14,18,22,26,30,34,38,42-undecaen-1-yl]oxy})phosphoryl]oxy})phosphoryl]oxy}-5-[(1-hydroxyethylidene)amino]-2-(hydroxymethyl)oxan-4-yl]oxy}-1-hydroxypropylidene)amino]-1-hydroxypropylidene]amino}butanoic acid
CAS: Not Available
Description: Undecaprenyl-diphospho-N-acetylmuramoyl-(N-acetylglucosamine)-L-alanyl-gamma-D-glutamyl-L-lysyl-D-alanyl-D-alanine is an intermediate in peptidoglycan synthesis. It is a substrate for the enzyme undecaprenyldiphospho-muramoylpentapeptide beta-N-acetylglucosaminyltransferase (murG). Peptidoglycan can be described as a fisherman's net that encloses bacteria. The mesh of the net is made of two segments of parallel, somewhat inextensible glycan threads, held together by two small elastic peptide crosslinks allowing the net to expand or shrink. The glycan moiety of the peptidoglycan is very uniform among all bacteria, and is made up of alternating β-1,4-linked N-acetylglucosamine and N-acetyl muramate residues, with an average chain lengthof 10 to 65 disaccharide units (depending on the organism). The peptidoglycan synthesis pathway starts in the cytoplasm, where in six steps the peptidoglycan precursor a UDP-N-acetylmuramoyl-pentapeptide is synthesized. This precursor is then attached to the memberane acceptor all-trans-undecaprenyl phosphate, generating a N-acetylmuramoyl-pentapeptide-diphosphoundecaprenol, also known as lipid I. Another transferase then adds UDP-N-acetyl-α-D-glucosamine, yielding the complete monomeric unit a lipid II, also known as lipid II. This final lipid intermediate is transferred by an as yet unknown mechanism through the membrane. The peptidoglycan monomers are then polymerized on the outside surface by glycosyltransferases, which form the linear glycan chains, and transpeptidases, which catalyze the formation of peptide crosslinks. Peptide crosslinks form between different parts of the peptides depending on the organism. For example, in Mycobacteria and in Pseudomonas aeruginosa most links form between the carboxyl group of the penultimate D-alanine (residue 4) of one peptide to the amino group at the D-center of meso-diaminopimelate (residue 3) of an adjacent peptide of a second glycan chain (as in Pseudomonas aeruginosa). The crosslinking reaction is catalyzed by transpeptidases and involves the cleavage of the D-alanyl-D-alanine bond of the donor peptide, providing the energy to drive the reaction. As a result, the peptides in the peptidoglycan polymers are one or two amino acids shorter than the peptides in the monomers.