|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001041 |
|---|
|
Identification |
|---|
| Name: |
Sirohydrochlorin |
|---|
| Description: | Sirohydrochlorin is a member of the chemical class known as Precorrins. These are intermediates formed by methylation at one or more of the four rings prior to the formation of the macrocyclic corrin ring. typhimurium, precorrin-2 is a precursor of both siroheme and B12. (PMID 8955319) |
|---|
|
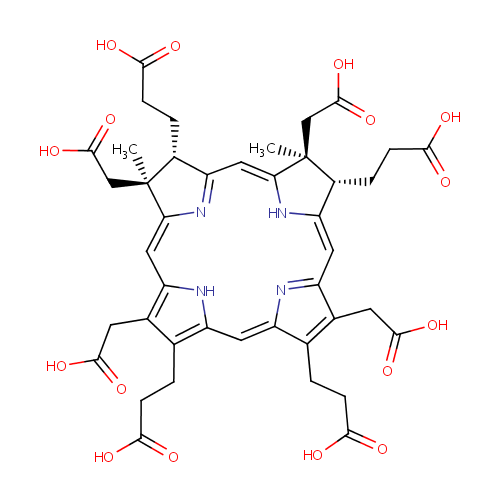
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C42H46N4O16 |
|---|
| Average Molecular Weight: |
862.8318 |
|---|
| Monoisotopic Molecular
Weight: |
862.290881444 |
|---|
| InChI Key: |
KWIZRXMMFRBUML-AHGFGAHVSA-N |
|---|
| InChI: | InChI=1S/C42H46N4O16/c1-41(17-39(59)60)23(5-9-35(51)52)29-14-27-21(11-37(55)56)19(3-7-33(47)48)25(43-27)13-26-20(4-8-34(49)50)22(12-38(57)58)28(44-26)15-31-42(2,18-40(61)62)24(6-10-36(53)54)30(46-31)16-32(41)45-29/h13-16,23-24,44-45H,3-12,17-18H2,1-2H3,(H,47,48)(H,49,50)(H,51,52)(H,53,54)(H,55,56)(H,57,58)(H,59,60)(H,61,62)/b25-13-,29-14-,31-15-,32-16-/t23-,24-,41+,42+/m1/s1 |
|---|
| CAS
number: |
65207-12-7 |
|---|
| IUPAC Name: | 3-[(4S,5S,9S,10S)-9,15,19-tris(2-carboxyethyl)-5,10,14,20-tetrakis(carboxymethyl)-5,10-dimethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1?,??1????.1??,???tetracosa-1(21),2,6,8(23),11,13,15,17,19-nonaen-4-yl]propanoic acid |
|---|
|
Traditional IUPAC Name: |
sirohydrochlorin |
|---|
| SMILES: | C[C@]1(CC(O)=O)[C@H](CCC(O)=O)\C2=C\C3=N\C(=C/C4=C(CCC(O)=O)C(CC(O)=O)=C(N4)\C=C4/N=C(/C=C1\N2)[C@@H](CCC(O)=O)[C@]4(C)CC(O)=O)\C(CCC(O)=O)=C3CC(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as tetrapyrroles and derivatives. These are polycyclic aromatic compounds containing four pyrrole rings joined by one-carbon units linking position 2 of one pyrrole ring to position 5 of the next. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Tetrapyrroles and derivatives |
|---|
| Sub Class | Not Available |
|---|
|
Direct Parent |
Tetrapyrroles and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Tetrapyrrole skeleton
- Substituted pyrrole
- Heteroaromatic compound
- Pyrrole
- Azacycle
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -8 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Membrane |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Porphyrin and chlorophyll metabolism pae00860
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Fazzio, T. G., Roth, J. R. (1996). "Evidence that the CysG protein catalyzes the first reaction specific to B12 synthesis in Salmonella typhimurium, insertion of cobalt." J Bacteriol 178:6952-6959. Pubmed: 8955319
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|