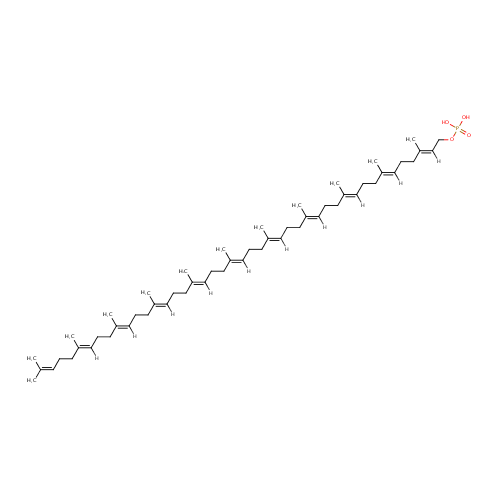
Undecaprenyl phosphate (PAMDB001054)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB001054 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Undecaprenyl phosphate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Undecaprenyl phosphate belongs to the class of Polyprenyl Phosphates. These are prenol lipids in which the phosphate group is linked to one end of the polyprenol moiety. (inferred from compound structure)C55-isoprenyl pyrophosphate (undecaprenyl pyrophosphate) is an essential molecule involved in construction of the bacterial peptidoglycan cell wall. (WikiPedia) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C55H91O4P | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 847.2824 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 846.665497912 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | UFPHFKCTOZIAFY-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C55H91O4P/c1-45(2)23-13-24-46(3)25-14-26-47(4)27-15-28-48(5)29-16-30-49(6)31-17-32-50(7)33-18-34-51(8)35-19-36-52(9)37-20-38-53(10)39-21-40-54(11)41-22-42-55(12)43-44-59-60(56,57)58/h23,25,27,29,31,33,35,37,39,41,43H,13-22,24,26,28,30,32,34,36,38,40,42,44H2,1-12H3,(H2,56,57,58) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 25126-51-6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | {[(2E,6E,10E,14E,18E,22E,26E,30E,34E,38E)-3,7,11,15,19,23,27,31,35,39,43-undecamethyltetratetraconta-2,6,10,14,18,22,26,30,34,38,42-undecaen-1-yl]oxy}phosphonic acid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | undecaprenyl phosphate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC(C)=CCCC(C)=CCCC(C)=CCCC(C)=CCCC(C)=CCCC(C)=CCCC(C)=CCCC(C)=CCCC(C)=CCCC(C)=CCCC(C)=CCOP(O)(O)=O | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as bactoprenol monophosphates. These are polyprenyl compounds consisting of a monophosphate group substituted by a bactoprenyl moiety. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Prenol lipids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Polyprenols | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Bactoprenol monophosphates | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Membrane | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | Water + Undecaprenyl diphosphate > Hydrogen ion + Phosphate + Undecaprenyl phosphate Undecaprenyl phosphate + UDP-N-Acetylmuramoyl-L-alanyl-D-glutamyl-6-carboxy-L-lysyl-D-alanyl-D-alanine > Undecaprenyl-diphospho-N-acetylmuramoyl-L-alanyl-D-glutamyl-meso-2,6-diaminopimeloyl-D-alanyl-D-alanine + Uridine 5'-monophosphate core oligosaccharide lipid A + Undecaprenyl diphosphate > core oligosaccharide lipid A diphosphate + Undecaprenyl phosphate Undecaprenyl phosphate + Uridine 5''-diphospho-{beta}-4-deoxy-4-formamido-L-arabinose > Undecaprenyl phosphate-4-amino-4-formyl-L-arabinose + Uridine 5'-diphosphate core oligosaccharide lipid A + undecaprenyl phosphate-4-amino-4-deoxy-L-arabinose > 4-Amino-4-deoxy-L-arabinose modified core oligosaccharide lipid A + Undecaprenyl phosphate Uridine diphosphate-N-acetylglucosamine + Undecaprenyl phosphate > Uridine 5'-monophosphate + Undecaprenyl-N-acetyl-alpha-D-glucosaminyl-pyrophosphate Undecaprenyl-N-acetyl-alpha-D-glucosaminyl-pyrophosphate + UDP-N-Acetyl-D-mannosaminouronate <> Hydrogen ion + Undecaprenyl phosphate + Uridine 5'-diphosphate | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||