Search Results for compounds
Searching compounds for
returned 4373 results.
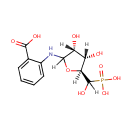
N-(5-Phospho-D-ribosyl)anthranilate (PAMDB001021)
IUPAC:
2-{[(3R,4S,5S)-3,4-dihydroxy-5-[hydroxy(phosphono)methyl]oxolan-2-yl]amino}benzoic acid
CAS: 4220-99-9
Description: N-(5-phospho-D-ribosyl)anthranilate is a member of the chemical class known as Pentoses. These are monosaccharides in which the carbohydrate moiety contains five carbon atoms. N-(5-Phospho-D-ribosyl)anthranilate is invovled in tryptophan biosynthesis. N-(5'-Phosphoribosyl)anthranilate isomerase-indole-3-glycerol-phosphate synthase from Pseudomonas aeruginosa is a monomeric bifunctional enzyme of Mr 49,500 that catalyzes two sequential reactions in the biosynthesis of tryptophan. (PMID 3303031) Phosphoribosyl anthranilate isomerase from Thermotoga maritima is an extremely stable and active homodimer. (PMID 8897600)
N-Acetyl-D-muramoate (PAMDB001023)
IUPAC:
(2R)-2-{[(3R,4R,5S,6R)-2,5-dihydroxy-3-[(1-hydroxyethylidene)amino]-6-(hydroxymethyl)oxan-4-yl]oxy}propanoic acid
CAS: 61633-75-8
Description: N-acetyl-D-muramoate is a member of the chemical class known as Sugar Acids and Derivatives. These are compounds containing a saccharide unit which bears a carboxylic acid group. UDP-N-acetylmuramic acid (UDP-MurNAc) is a precursor for peptidoglycan biosynthesis in bacteria. (PMID 14659546) Peptidoglycan is the major constituent of the cell wall, which is comprised of backbone repeats of N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM). (PMID 18700763) UDP-N-acetylmuramic acid (UDP-MurNAc) is a potent inhibitor of MurA (enolpyruvyl-UDP-GlcNAc synthase). (PMID 15751977) The bacterial cell wall is a polymer consisting of alternating N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid (MurNAc) units, cross-linked via peptides appended to MurNAc. (PMID 12369847). The peptidoglycan synthesis pathway starts at the cytoplasm, where in six steps the peptidoglycan precursor a UDP-N-acetylmuramoyl-pentapeptide is synthesized. This precursor is then attached to the memberane acceptor all-trans-undecaprenyl phosphate, generating a N-acetylmuramoyl-pentapeptide-diphosphoundecaprenol, also known as lipid I. Another transferase then adds UDP-N-acetyl-alpha-D-glucosamine, yielding the complete monomeric unit a lipid , also known as lipid . This final lipid intermediate is transferred through the membrane. The peptidoglycan monomers are then polymerized on the outside surface by glycosyltransferases, which form the linear glycan chains, and transpeptidases, which catalyze the formation of peptide crosslinks.
N-Acetylmuramoyl-Ala (PAMDB001025)
IUPAC:
(2R)-2-{[(2R)-2-{[(2S,3R,4R,5S,6R)-2,5-dihydroxy-3-[(1-hydroxyethylidene)amino]-6-(hydroxymethyl)oxan-4-yl]oxy}-1-hydroxypropylidene]amino}propanoic acid
CAS: Not Available
Description: N-acetylmuramoyl-Ala is a member of the chemical class known as Hexoses. These are monosaccharides in which the sugar unit is a hexose. It is a key component of peptidoglycan synthesis. The peptidoglycan synthesis pathway starts at the cytoplasm, where in six steps the peptidoglycan precursor a UDP-N-acetylmuramoyl-pentapeptide is synthesized. This precursor is then attached to the memberane acceptor all-trans-undecaprenyl phosphate, generating a N-acetylmuramoyl-pentapeptide-diphosphoundecaprenol, also known as lipid I. Another transferase then adds UDP-N-acetyl-alpha-D-glucosamine, yielding the complete monomeric unit a lipid , also known as lipid . This final lipid intermediate is transferred through the membrane. The peptidoglycan monomers are then polymerized on the outside surface by glycosyltransferases, which form the linear glycan chains, and transpeptidases, which catalyze the formation of peptide crosslinks.
Precorrin 2 (PAMDB001029)
IUPAC:
(4S,5S,9S,10S)-4,9,15,19-tetrakis(2-carboxyethyl)-5,10,14,20-tetrakis(carboxymethyl)-5,10-dimethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1?,??1????.1??,???tetracosa-1(20),2,6(24),7,11(23),12,14,16(22),18-nonaen-22-ium-21-ide
CAS: 82542-92-5
Description: Precorrin 2 is a member of the chemical class known as Tetrapyrroles and Derivatives. These are polycyclic aromatic compounds containing four pyrrole rings joined by one-carbon units linking position 2 of one pyrrole ring to position 5 of the next. Precorrin 2 is invovled in Proto- and siroheme biosynthesis. Precorrin-2 is a precursor of both siroheme and B12. (PMID 8955319)
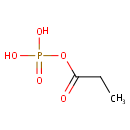
Propanoyl phosphate (PAMDB001030)
IUPAC:
(propanoyloxy)phosphonic acid
CAS: 121-69-7
Description: Propanoyl phosphate is an alkylphosphate. It is generated during the course of threonine degradation via propanoyl-CoA. The first reaction in the anaerobic threonine dehydratase pathway is catalyzed by catabolic threonine dehydratase which degrades threonine to 2-oxobutanoate (alpha-ketobutyrate) and ammonia. The 2-oxobutanoate then undergoes lyase cleavage with the addition of coenzyme A to form propanoyl-CoA and formate. Once propanoyl-CoA is formed, it is processed via propionyl-phosphate to propionate in a reaction sequence that produces ATP. Acetate kinase AckA can also utilize propionate as a substrate in the final reaction. The enzymes in this pathway are also able to process L-serine, with pyruvate as the final product [EcoCyc].
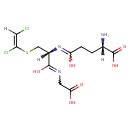
S-(1,2-Dichlorovinyl)glutathione (PAMDB001032)
IUPAC:
(2S)-2-amino-4-{[(1R)-1-[(carboxymethyl)-C-hydroxycarbonimidoyl]-2-{[(E)-1,2-dichloroethenyl]sulfanyl}ethyl]-C-hydroxycarbonimidoyl}butanoic acid
CAS: 96614-59-4
Description: S-(1,2-dichlorovinyl)glutathione is a member of the chemical class known as Peptides. These are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another.
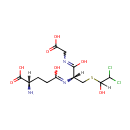
S-(2-Chloroacetyl)glutathione (PAMDB001033)
IUPAC:
(2S)-2-amino-4-{[(1R)-1-[(carboxymethyl)-C-hydroxycarbonimidoyl]-2-[(2-chloroacetyl)sulfanyl]ethyl]-C-hydroxycarbonimidoyl}butanoic acid
CAS: 113668-38-5
Description: S-(2-chloroacetyl)glutathione is a member of the chemical class known as Peptides. These are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another.
S-(2,2-Dichloro-1-hydroxy)ethyl glutathione (PAMDB001034)
IUPAC:
(2S)-2-amino-4-{[(1R)-1-[(carboxymethyl)-C-hydroxycarbonimidoyl]-2-[(2,2-dichloro-1-hydroxyethyl)sulfanyl]ethyl]-C-hydroxycarbonimidoyl}butanoic acid
CAS: Not Available
Description: S-(2,2-dichloro-1-hydroxy)ethyl glutathione belongs to the class of Peptides. These are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another. (inferred from compound structure)
S-(Formylmethyl)glutathione (PAMDB001035)
IUPAC:
(2S)-2-amino-4-{[(1R)-1-[(carboxymethyl)-C-hydroxycarbonimidoyl]-2-[(2-oxoethyl)sulfanyl]ethyl]-C-hydroxycarbonimidoyl}butanoic acid
CAS: Not Available
Description: S-(formylmethyl)glutathione belongs to the class of Peptides. These are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another. (inferred from compound structure)
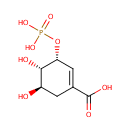
Shikimate 3-phosphate (PAMDB001039)
IUPAC:
(3R,4S,5R)-4,5-dihydroxy-3-(phosphonooxy)cyclohex-1-ene-1-carboxylic acid
CAS: Not Available
Description: Shikimate 3-phosphate is a member of the chemical class known as Organophosphate Esters. These are organic compounds containing phosphoric acid ester functional group. Shikimate 3-phosphate is involved in the shikimate pathway. The shikimate pathway enzyme 5-enolpyruvyl shikimate-3-phosphate synthase (EPSP synthase) has received attention in the past because it is the target of the broad-spectrum herbicide glyphosate. (PMID 16225867) The enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) catalyzes the penultimate step of the shikimate pathway and is the target of the broad-spectrum herbicide glyphosate. (PMID 15736934)