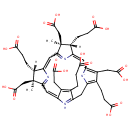
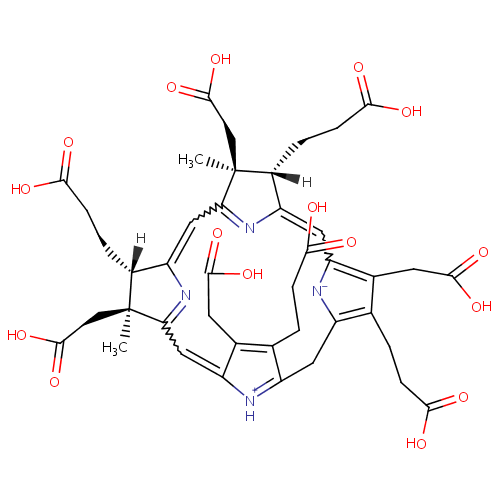
Precorrin 2 (PAMDB001029)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB001029 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | Precorrin 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Precorrin 2 is a member of the chemical class known as Tetrapyrroles and Derivatives. These are polycyclic aromatic compounds containing four pyrrole rings joined by one-carbon units linking position 2 of one pyrrole ring to position 5 of the next. Precorrin 2 is invovled in Proto- and siroheme biosynthesis. Precorrin-2 is a precursor of both siroheme and B12. (PMID 8955319) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C42H46N4O16 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 862.8318 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 862.290881444 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | KVFNZYKFDBWLHT-ZTKUHGNGSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C42H46N4O16/c1-41(17-39(59)60)23(5-9-35(51)52)29-14-27-21(11-37(55)56)19(3-7-33(47)48)25(43-27)13-26-20(4-8-34(49)50)22(12-38(57)58)28(44-26)15-31-42(2,18-40(61)62)24(6-10-36(53)54)30(46-31)16-32(41)45-29/h14-16,23-24H,3-13,17-18H2,1-2H3,(H9,43,44,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62)/t23-,24-,41+,42+/m1/s1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 82542-92-5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (4S,5S,9S,10S)-4,9,15,19-tetrakis(2-carboxyethyl)-5,10,14,20-tetrakis(carboxymethyl)-5,10-dimethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1?,??1????.1??,???tetracosa-1(20),2,6(24),7,11(23),12,14,16(22),18-nonaen-22-ium-21-ide | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | (4S,5S,9S,10S)-4,9,15,19-tetrakis(2-carboxyethyl)-5,10,14,20-tetrakis(carboxymethyl)-5,10-dimethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1?,??1????.1??,???tetracosa-1(20),2,6(24),7,11(23),12,14,16(22),18-nonaen-22-ium-21-ide | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | [H][C@@]1(CCC(O)=O)C2=CC3=C(CC(O)=O)C(CCC(O)=O)=C(CC4=[NH+]C(=CC5=NC(=CC(=N2)[C@@]1(C)CC(O)=O)[C@@]([H])(CCC(O)=O)[C@]5(C)CC(O)=O)C(CC(O)=O)=C4CCC(O)=O)[N-]3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as tetrapyrroles and derivatives. These are polycyclic aromatic compounds containing four pyrrole rings joined by one-carbon units linking position 2 of one pyrrole ring to position 5 of the next. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Tetrapyrroles and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Tetrapyrroles and derivatives | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic heteropolycyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -8 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 2 S-Adenosylmethionine + Uroporphyrinogen III >2 S-Adenosylhomocysteine + Precorrin 2 + Hydrogen ion Precorrin 2 + NAD > Hydrogen ion + NADH + Sirohydrochlorin 2 S-Adenosylmethionine + Uroporphyrinogen III <>2 S-Adenosylhomocysteine + Precorrin 2 S-Adenosylmethionine + precorrin-1 > S-Adenosylhomocysteine + Precorrin 2 2 S-Adenosylmethionine + Uroporphyrinogen III + Precorrin-1 <>2 S-Adenosylhomocysteine + Precorrin 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||