Search Results for compounds
Searching compounds for
returned 4373 results.
Displaying compounds 4081 - 4090 of
4373 in total
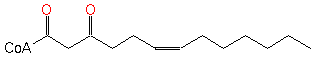
6-cis, 3-oxo-tridecenoyl-CoA (PAMDB120364)
IUPAC:
Not Available
CAS: Not Available
Description: Not Available
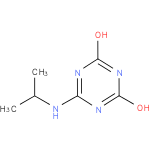
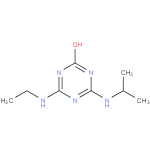
N-isopropylammelide (PAMDB120365)
IUPAC:
6-(isopropylamino)-1,3,5-triazine-2,4-diol
CAS: Not Available
Description: A dihydroxy-1,3,5-triazine consisting of ammelide bearing an N-isopropyl substituent.
palmitoleate (PAMDB120366)
IUPAC:
(9Z)-hexadec-9-enoate
CAS: 373-49-9
Description: A hexadec-9-enoate that is the conjugate base of palmitoleic acid; major species at pH 7.3.
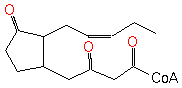
OPC4-3-ketoacyl-CoA (PAMDB120367)
IUPAC:
Not Available
CAS: Not Available
Description: Not Available
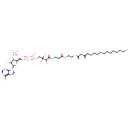
3-oxo-palmitoyl-CoA (PAMDB120369)
IUPAC:
3'- phosphonatoadenosine 5'-
phosphonatoadenosine 5'- {3-
{3- [(3R)-
[(3R)- 3-
3- hydroxy-
hydroxy- 2,2-
2,2- dimethyl-
dimethyl- 4-
4- oxo-
oxo- 4-
4- {[3-
{[3- oxo-
oxo- 3-
3- ({2-
({2- [(3-
[(3- oxohexadecanoyl)sulfanyl]ethyl}amino)propyl]amino}butyl] diphosphate}
oxohexadecanoyl)sulfanyl]ethyl}amino)propyl]amino}butyl] diphosphate}
CAS: 34619-89-1
Description: An acyl-CoA(4−) arising from deprotonation of the phosphate and diphosphate OH groups of 3-oxopalmitoyl-CoA.
hydroxyatrazine (PAMDB120370)
IUPAC:
4-(ethylamino)-6-(isopropylamino)-1,3,5-triazin-2-ol
CAS: Not Available
Description: A monohydroxy-1,3,5-triazine that is atrazine in which the chloro group has been replaced by a hydroxy group.
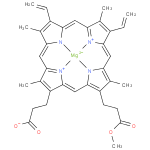
magnesium-protoporphyrin IX 13-monomethyl ester (PAMDB120371)
IUPAC:
Not Available
CAS: Not Available
Description: The conjugate base of magnesium protoporphyrin 13-monomethyl ester, formed by deprotonation of the carboxyethyl group at C-17. It is the principal species at pH 7.3.
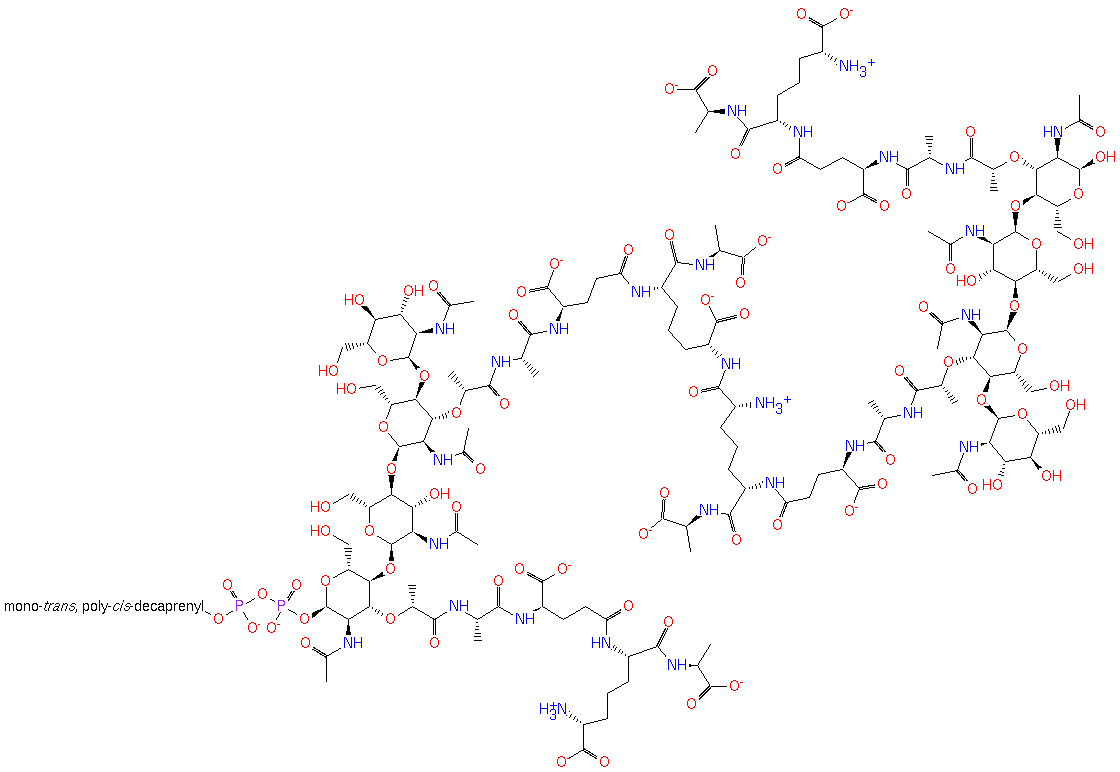
a peptidoglycan with L,D cross-links (mycobacteria like) (PAMDB120372)
IUPAC:
Not Available
CAS: Not Available
Description: Not Available
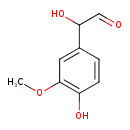
3-methoxy-4-hydroxyphenylglycolaldehyde (PAMDB120374)
IUPAC:
2-hydroxy-2-(4-hydroxy-3-methoxyphenyl)acetaldehyde
CAS: 17592-23-3
Description: 3-Methoxy-4-hydroxyphenylglycolaldehyde is the monoamine oxidase (MAO) aldehyde metabolite of metanephrine. Metanephrine is an O-methylated metabolite formed by catechol-O-methyltransferase (COMT) from epinephrine. Catecholamines play an important role in platelet activation and aggregation, epinephrine being the most potent one. Catecholamines are substantially increased during stress, exercise or smoking and could result in clinically important platelet activation if their action was not rapidly regulated. The inhibitory effects of methoxy phenolic derivatives on epinephrine-induced platelet aggregation may possibly be attributed to their free radical scavenging properties. There is substantial evidence to conclude that an internal rapid autoregulation of epinephrine-induced platelet aggregation, caused by its metabolic degradation products, takes place in vivo. (PMID: 11958479 , 9706478 ).