|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120370 |
|---|
|
Identification |
|---|
| Name: |
hydroxyatrazine |
|---|
| Description: | A monohydroxy-1,3,5-triazine that is atrazine in which the chloro group has been replaced by a hydroxy group. |
|---|
|
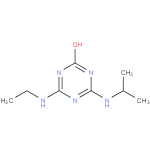
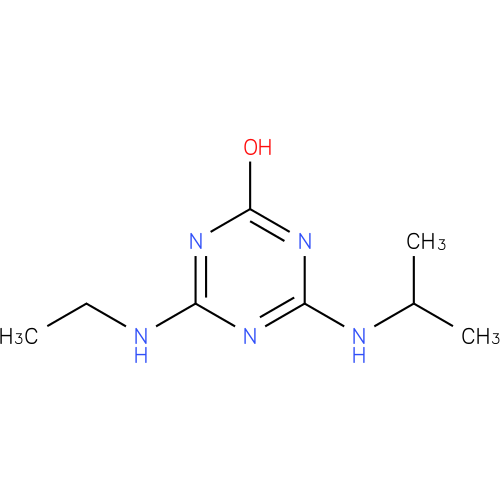
Structure |
|
|---|
| Synonyms: | - 2-hydroxyatrazine
- 4-(Ethylamino)-2-hydroxy-6-(isopropylamino)-1,3,5-triazine
- 4-(ethylamino)-6-(isopropylamino)-s-triazin-2-ol
- Hydroxyatrazine
- hydroxyatrazine
- hydroxydechloroatrazine
|
|---|
|
Chemical Formula: |
C8H15N5O |
|---|
| Average Molecular Weight: |
197.239 |
|---|
| Monoisotopic Molecular
Weight: |
197.12766 |
|---|
| InChI Key: |
NFMIMWNQWAWNDW-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C8H15N5O/c1-4-9-6-11-7(10-5(2)3)13-8(14)12-6/h5H,4H2,1-3H3,(H3,9,10,11,12,13,14) |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 4-(ethylamino)-6-(isopropylamino)-1,3,5-triazin-2-ol |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CCNC1(=NC(NC(C)C)=NC(O)=N1) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as 1,3,5-triazines. These are compounds containing a triazine ring, which is a heterocyclic ring, similar to the six-member benzene ring but with three carbons replaced by nitrogen atoms, at ring positions 1, 3, and 5. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Triazines |
|---|
| Sub Class | 1,3,5-triazines |
|---|
|
Direct Parent |
1,3,5-triazines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 1,3,5-triazine
- Heteroaromatic compound
- Azacycle
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
- diamino-1,3,5-triazine, monohydroxy-1,3,5-triazine (CHEBI:18316)
- a small molecule (HYDROXYATRAZINE)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- atrazine degradation I (aerobic)P141-PWY
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Baranda AB, Barranco A, de Marañón IM (2012)Fast atrazine photodegradation in water by pulsed light technology. Water research 46, Pubmed: 22153354
- Panuwet P, Restrepo PA, Magsumbol M, Jung KY, Montesano MA, Needham LL, Barr DB (2010)An improved high-performance liquid chromatography-tandem mass spectrometric method to measure atrazine and its metabolites in human urine. Journal of chromatography. B, Analytical technologies in the biomedical and life sciences 878, Pubmed: 20299293
- Kolekar PD, Phugare SS, Jadhav JP (2014)Biodegradation of atrazine by Rhodococcus sp. BCH2 to N-isopropylammelide with subsequent assessment of toxicity of biodegraded metabolites. Environmental science and pollution research international 21, Pubmed: 24062064
- Seybold CA, Mersie W, McNamee C (2001)Anaerobic degradation of atrazine and metolachlor and metabolite formation in wetland soil and water microcosms. Journal of environmental quality 30, Pubmed: 11476505
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|