|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120365 |
|---|
|
Identification |
|---|
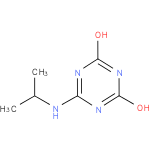
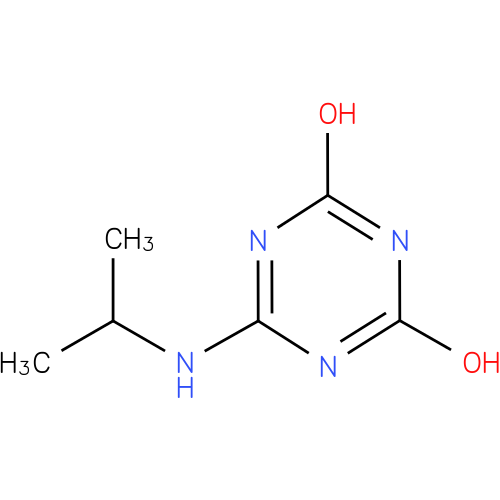
| Name: |
N-isopropylammelide |
|---|
| Description: | A dihydroxy-1,3,5-triazine consisting of ammelide bearing an N-isopropyl substituent. |
|---|
|
Structure |
|
|---|
| Synonyms: | - N-Isopropylammelide
- N-isopropylammelide
|
|---|
|
Chemical Formula: |
C6H10N4O2 |
|---|
| Average Molecular Weight: |
170.171 |
|---|
| Monoisotopic Molecular
Weight: |
170.08037 |
|---|
| InChI Key: |
DBFMBHXVWIURSV-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C6H10N4O2/c1-3(2)7-4-8-5(11)10-6(12)9-4/h3H,1-2H3,(H3,7,8,9,10,11,12) |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 6-(isopropylamino)-1,3,5-triazine-2,4-diol |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CC(NC1(N=C(O)N=C(N=1)O))C |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as n-aliphatic s-triazines. These are n-aliphatic amine derivatives of 1,3,5-triazines. 1,3,5-triazines are aromatic compounds having three nitrogen ring atoms at the 1-, 3-, and 5- positions. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Triazines |
|---|
| Sub Class | Aminotriazines |
|---|
|
Direct Parent |
N-aliphatic s-triazines |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Secondary aliphatic/aromatic amine
- Triazinone
- N-aliphatic s-triazine
- 1,3,5-triazine
- Heteroaromatic compound
- Urea
- Azacycle
- Secondary amine
- Organic oxide
- Hydrocarbon derivative
- Organonitrogen compound
- Amine
- Organooxygen compound
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aromatic heteromonocyclic compounds |
|---|
| External Descriptors |
- dihydroxy-1,3,5-triazine, monoamino-1,3,5-triazine (CHEBI:17247)
- a small molecule (N-ISOPROPYLAMMELIDE)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
- atrazine degradation I (aerobic)P141-PWY
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Seffernick JL, Aleem A, Osborne JP, Johnson G, Sadowsky MJ, Wackett LP (2007)Hydroxyatrazine N-ethylaminohydrolase (AtzB): an amidohydrolase superfamily enzyme catalyzing deamination and dechlorination. Journal of bacteriology 189, Pubmed: 17660279
- Devers M, Rouard N, Martin-Laurent F (2008)Fitness drift of an atrazine-degrading population under atrazine selection pressure. Environmental microbiology 10, Pubmed: 18237303
- Liu X, Parales RE (2009)Bacterial chemotaxis to atrazine and related s-triazines. Applied and environmental microbiology 75, Pubmed: 19581468
- Sadowsky MJ, Tong Z, de Souza M, Wackett LP (1998)AtzC is a new member of the amidohydrolase protein superfamily and is homologous to other atrazine-metabolizing enzymes. Journal of bacteriology 180, Pubmed: 9422605
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|