Search Results for compounds
Searching compounds for
returned 4373 results.
Displaying compounds 3961 - 3970 of
4373 in total
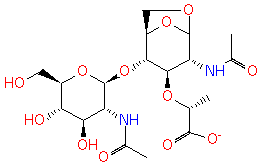
N-acetyl-β-D-glucosamine(anhydrous)-N-acetylmuramate (PAMDB120237)
IUPAC:
Not Available
CAS: Not Available
Description: Not Available
3-demethylubiquinol-7 (PAMDB120238)
IUPAC:
Not Available
CAS: Not Available
Description: Not Available
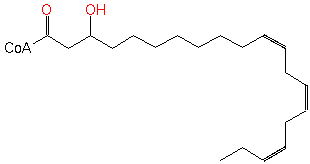
3-hydroxy-eicosatrienoyl-CoA (PAMDB120239)
IUPAC:
Not Available
CAS: Not Available
Description: Not Available
maltoheptaose (PAMDB120241)
IUPAC:
α- D-
D- glucopyranosyl-
glucopyranosyl- (1→4)-
(1→4)- α-
α- D-
D- glucopyranosyl-
glucopyranosyl- (1→4)-
(1→4)- α-
α- D-
D- glucopyranosyl-
glucopyranosyl- (1→4)-
(1→4)- α-
α- D-
D- glucopyranosyl-
glucopyranosyl- (1→4)-
(1→4)- α-
α- D-
D- glucopyranosyl-
glucopyranosyl- (1→4)-
(1→4)- α-
α- D-
D- glucopyranosyl-
glucopyranosyl- (1→4)-
(1→4)- α-
α- D-
D- glucopyranose
glucopyranose
CAS: Not Available
Description: A maltoheptaose heptasaccharide in which the glucose residue at the reducing end is in the pyranose ring form and has α configuration at the anomeric carbon atom.
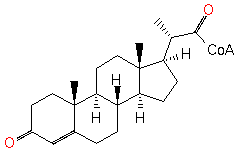
3-oxo-23,24-bisnorchol-4-en-22-oyl-CoA (PAMDB120242)
IUPAC:
Not Available
CAS: Not Available
Description: Not Available
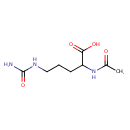
N-acetyl-L-citrulline (PAMDB120243)
IUPAC:
(2S)-2-acetamido-5-(carbamoylamino)pentanoic acid
CAS: 33965-42-3
Description: The L-enantiomer of N-acetylcitrulline.
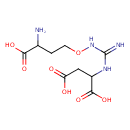
canavaninosuccinate (PAMDB120244)
IUPAC:
2-[3-(3-amino-3-carboxypropoxy)carbamimidamido]butanedioic acid
CAS: 56073-32-6
Description: Canavaninosuccinate is formed from ureidohomoserine and aspartate by a human or bovine liver extract that had high argininosuccinate synthetase (EC 6.3.4.5) activity, and the subsequent formation of guanidinosuccinate is done by reductive cleavage. In the presence of ATP the optimum pH for the synthetic reaction is 8.4. This reaction can be carried out in either a tris(hydroxymethyl)aminomethane or borate buffer. Subsequent addition of dithiothreitol in the presence of Fe2+ resulted in the cleavage of some of the synthesized canavaninosuccinate to form guanidinosuccinate and homoserine. Synthesis of canavaninosuccinate was strongly inhibited by added argininosuccinate, less so by canavaninosuccinate, arginine, canavanine, glycine, or 2,3-dimercaptopropanol. All the reactions, starting with canavaninosuccinate and down to the formation of guanidinoacetate and guanidinosuccinate have been demonstrated in human tissue ( (PMID: 241511 ).
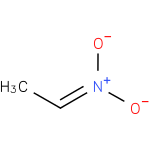
ethylnitronate (PAMDB120245)
IUPAC:
[ethylidene(oxido)-λ5-azanyl]oxidanide
CAS: Not Available
Description: A nitrogen oxoanion arising from deprotonation of nitroethane.
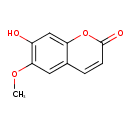
scopoletin (PAMDB120246)
IUPAC:
7-hydroxy-6-methoxy-2H-chromen-2-one
CAS: 92-61-5
Description: A hydroxycoumarin that is umbelliferone bearing a methoxy substituent at position 6.