|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120241 |
|---|
|
Identification |
|---|
| Name: |
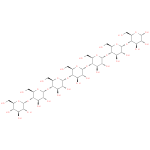
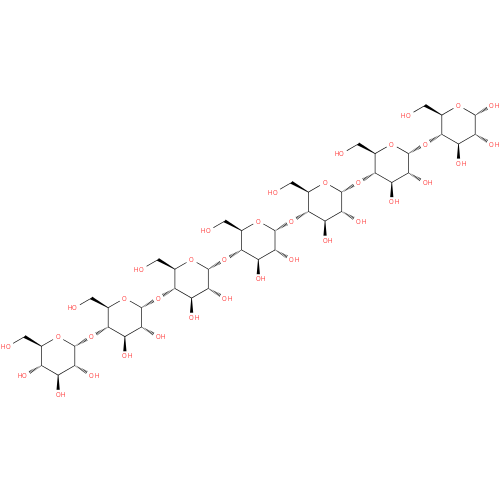
maltoheptaose |
|---|
| Description: | A maltoheptaose heptasaccharide in which the glucose residue at the reducing end is in the pyranose ring form and has α configuration at the anomeric carbon atom. |
|---|
|
Structure |
|
|---|
| Synonyms: | - α-
 D- D- Glc- Glc- (1→4)- (1→4)- α- α- D- D- Glc- Glc- (1→4)- (1→4)- α- α- D- D- Glc- Glc- (1→4)- (1→4)- α- α- D- D- Glc- Glc- (1→4)- (1→4)- α- α- D- D- Glc- Glc- (1→4)- (1→4)- α- α- D- D- Glc- Glc- (1→4)- (1→4)- α- α- D- D- Glc Glc - α-
 D- D- Glcp- Glcp- (1→4)- (1→4)- α- α- D- D- Glcp- Glcp- (1→4)- (1→4)- α- α- D- D- Glcp- Glcp- (1→4)- (1→4)- α- α- D- D- Glcp- Glcp- (1→4)- (1→4)- α- α- D- D- Glcp- Glcp- (1→4)- (1→4)- α- α- D- D- Glcp- Glcp- (1→4)- (1→4)- α- α- D- D- Glcp Glcp - α-
 D- D- glucosyl- glucosyl- (1→4)- (1→4)- α- α- D- D- glucosyl- glucosyl- (1→4)- (1→4)- α- α- D- D- glucosyl- glucosyl- (1→4)- (1→4)- α- α- D- D- glucosyl- glucosyl- (1→4)- (1→4)- α- α- D- D- glucosyl- glucosyl- (1→4)- (1→4)- α- α- D- D- glucosyl- glucosyl- (1→4)- (1→4)- α- α- D- D- glucose glucose - α-maltoheptaose
|
|---|
|
Chemical Formula: |
C42H72O36 |
|---|
| Average Molecular Weight: |
1153.009 |
|---|
| Monoisotopic Molecular
Weight: |
1152.3804 |
|---|
| InChI Key: |
BNABBHGYYMZMOA-QJBBZCPBSA-N |
|---|
| InChI: | InChI=1S/C42H72O36/c43-1-8-15(50)16(51)24(59)37(67-8)74-31-10(3-45)69-39(26(61)18(31)53)76-33-12(5-47)71-41(28(63)20(33)55)78-35-14(7-49)72-42(29(64)22(35)57)77-34-13(6-48)70-40(27(62)21(34)56)75-32-11(4-46)68-38(25(60)19(32)54)73-30-9(2-44)66-36(65)23(58)17(30)52/h8-65H,1-7H2/t8-,9-,10-,11-,12-,13-,14-,15-,16+,17-,18-,19-,20-,21-,22-,23-,24-,25-,26-,27-,28-,29-,30-,31-,32-,33-,34-,35-,36?,37-,38-,39-,40-,41-,42-/m1/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | α- D- D- glucopyranosyl- glucopyranosyl- (1→4)- (1→4)- α- α- D- D- glucopyranosyl- glucopyranosyl- (1→4)- (1→4)- α- α- D- D- glucopyranosyl- glucopyranosyl- (1→4)- (1→4)- α- α- D- D- glucopyranosyl- glucopyranosyl- (1→4)- (1→4)- α- α- D- D- glucopyranosyl- glucopyranosyl- (1→4)- (1→4)- α- α- D- D- glucopyranosyl- glucopyranosyl- (1→4)- (1→4)- α- α- D- D- glucopyranose glucopyranose |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | C(O)C1(C(O)C(O)C(O)C(O1)OC2(C(O)C(O)C(OC(CO)2)OC3(C(O)C(O)C(OC(CO)3)OC7(C(O)C(O)C(OC6(C(O)C(O)C(OC4(C(O)C(O)C(OC(CO)4)OC5(C(O)C(O)C(O)OC(CO)5)))OC(CO)6))OC(CO)7)))) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as oligosaccharides. These are carbohydrates made up of 3 to 10 monosaccharide units linked to each other through glycosidic bonds. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
|
Class |
Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
|
Direct Parent |
Oligosaccharides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Oligosaccharide
- O-glycosyl compound
- Glycosyl compound
- Oxane
- Secondary alcohol
- Hemiacetal
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Acetal
- Hydrocarbon derivative
- Primary alcohol
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
- an oligosaccharide (CPD0-1133)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- van de Weerd R, Berbís MA, Sparrius M, Maaskant JJ, Boot M, Paauw NJ, de Vries N, Boon L, Baba O, Cañada FJ, Geurtsen J, Jiménez-Barbero J, Appelmelk BJ (2015)A murine monoclonal antibody to glycogen: characterization of epitope-fine specificity by saturation transfer difference (STD) NMR spectroscopy and its use in mycobacterial capsular a-glucan research. Chembiochem : a European journal of chemical biology 16, Pubmed: 25766777
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|