|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120244 |
|---|
|
Identification |
|---|
| Name: |
canavaninosuccinate |
|---|
| Description: | Canavaninosuccinate is formed from ureidohomoserine and aspartate by a human or bovine liver extract that had high argininosuccinate synthetase (EC 6.3.4.5) activity, and the subsequent formation of guanidinosuccinate is done by reductive cleavage. In the presence of ATP the optimum pH for the synthetic reaction is 8.4. This reaction can be carried out in either a tris(hydroxymethyl)aminomethane or borate buffer. Subsequent addition of dithiothreitol in the presence of Fe2+ resulted in the cleavage of some of the synthesized canavaninosuccinate to form guanidinosuccinate and homoserine. Synthesis of canavaninosuccinate was strongly inhibited by added argininosuccinate, less so by canavaninosuccinate, arginine, canavanine, glycine, or 2,3-dimercaptopropanol. All the reactions, starting with canavaninosuccinate and down to the formation of guanidinoacetate and guanidinosuccinate have been demonstrated in human tissue ( (PMID: 241511 ). |
|---|
|
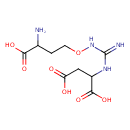
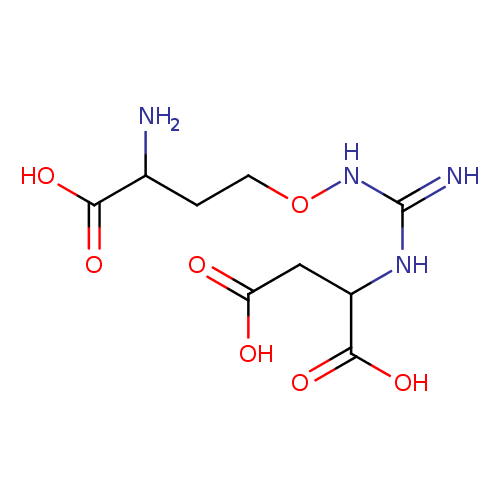
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C9H15N4O7 |
|---|
| Average Molecular Weight: |
291.24 |
|---|
| Monoisotopic Molecular
Weight: |
294.11755 |
|---|
| InChI Key: |
SGYMGUGIGTWWLU-UHFFFAOYSA-M |
|---|
| InChI: | InChI=1S/C9H16N4O7/c10-4(7(16)17)1-2-20-13-9(11)12-5(8(18)19)3-6(14)15/h4-5H,1-3,10H2,(H,14,15)(H,16,17)(H,18,19)(H3,11,12,13)/p-1 |
|---|
| CAS
number: |
56073-32-6 |
|---|
| IUPAC Name: | 2-[3-(3-amino-3-carboxypropoxy)carbamimidamido]butanedioic acid |
|---|
|
Traditional IUPAC Name: |
2-[3-(3-amino-3-carboxypropoxy)carbamimidamido]butanedioic acid |
|---|
| SMILES: | C(ONC(=[N+])NC(CC(=O)[O-])C(=O)[O-])CC(C([O-])=O)[N+] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as aspartic acid and derivatives. These are compounds containing an aspartic acid or a derivative thereof resulting from reaction of aspartic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
Aspartic acid and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Aspartic acid or derivatives
- Alpha-amino acid
- Tricarboxylic acid or derivatives
- Guanidine
- Amino acid
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboximidamide
- Carboxylic acid
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Amine
- Organopnictogen compound
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | Not Available |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Koller A, Aldwin L, Natelson S: Hepatic synthesis of canavaninosuccinate from ureidohomoserine and aspartate, and its conversion to guanidinosuccinate. Clin Chem. 1975 Nov;21(12):1777-82. [241511 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|