|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB120243 |
|---|
|
Identification |
|---|
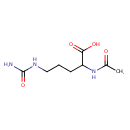
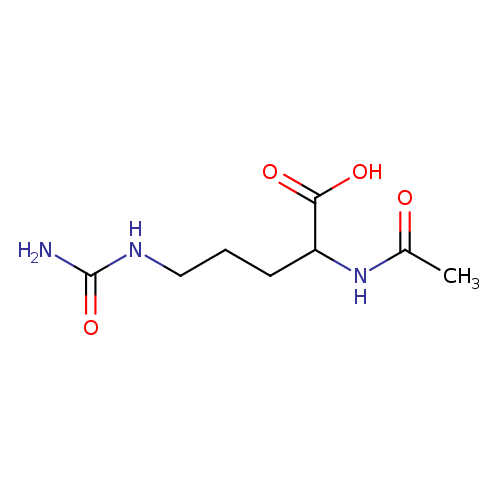
| Name: |
N-acetyl-L-citrulline |
|---|
| Description: | The L-enantiomer of N-acetylcitrulline. |
|---|
|
Structure |
|
|---|
| Synonyms: | - (2S)-2-(acetylamino)-5-[(aminocarbonyl)amino]pentanoic acid
- (S)-2-ACETAMIDO-5-UREIDOPENTANOIC ACID
|
|---|
|
Chemical Formula: |
C8H14N3O4 |
|---|
| Average Molecular Weight: |
216.216 |
|---|
| Monoisotopic Molecular
Weight: |
217.10626 |
|---|
| InChI Key: |
WMQMIOYQXNRROC-LURJTMIESA-M |
|---|
| InChI: | InChI=1S/C8H15N3O4/c1-5(12)11-6(7(13)14)3-2-4-10-8(9)15/h6H,2-4H2,1H3,(H,11,12)(H,13,14)(H3,9,10,15)/p-1/t6-/m0/s1 |
|---|
| CAS
number: |
33965-42-3 |
|---|
| IUPAC Name: | (2S)-2-acetamido-5-(carbamoylamino)pentanoic acid |
|---|
|
Traditional IUPAC Name: |
N-acetylcitrulline |
|---|
| SMILES: | CC(=O)NC(C([O-])=O)CCCNC(=O)N |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as n-acyl-alpha amino acids. These are compounds containing an alpha amino acid which bears an acyl group at its terminal nitrogen atom. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic acids and derivatives |
|---|
| Sub Class | Carboxylic acids and derivatives |
|---|
|
Direct Parent |
N-acyl-alpha amino acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- N-acyl-alpha-amino acid
- Fatty acid
- Acetamide
- Carboxamide group
- Secondary carboxylic acid amide
- Urea
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Carbonyl group
- Organic nitrogen compound
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Engelke UF, Liebrand-van Sambeek ML, de Jong JG, Leroy JG, Morava E, Smeitink JA, Wevers RA: N-acetylated metabolites in urine: proton nuclear magnetic resonance spectroscopic study on patients with inborn errors of metabolism. Clin Chem. 2004 Jan;50(1):58-66. Epub 2003 Nov 18. [14633929 ]
|
|---|
| Synthesis Reference: |
Shi, Dashuang; Morizono, Hiroki; Yu, Xiaolin; Roth, Lauren; Caldovic, Ljubica; Allewell, Norma M.; Malamy, Michael H.; Tuchman, Mendel. Crystal Structure of N-Acetylornithine Transcarbamylase from Xanthomonas campestris: A novel enzyme in a new arginine biosynthetic pathway found in several eubacteria. Journal of Biological Chemistry (2005), 280(15), 14366-14369. (Biosynthetic preparation) |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|