Search Results for compounds
Searching compounds for
returned 4373 results.
Displaying compounds 3341 - 3350 of
4373 in total
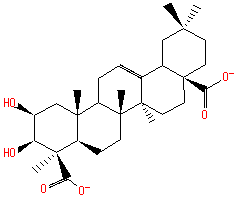
hederagenin (PAMDB110357)
IUPAC:
(3β)-3,23-dihydroxyolean-12-en-28-oic acid
CAS: Not Available
Description: A sapogenin that is olean-12-en-28-oic acid substituted by hydroxy groups at positions 3 and 23 (the 3β stereoisomer).
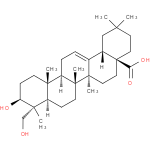
soyasapogenol B (PAMDB110358)
IUPAC:
(3β,22β)-olean-12-ene-3,22,24-triol
CAS: 595-15-3
Description: A pentacyclic triterpenoid that is oleanane containing a double bond between positions 12 and 13 and substituted by hydroxy groups at the 3β, 22β and 24-positions.
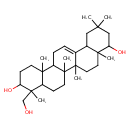
soyasapogenol E (PAMDB110359)
IUPAC:
(3β)-3,24-dihydroxyolean-12-en-22-one
CAS: 6750-59-0
Description: A pentacyclic triterpenoid that is oleanane containing a double bond between positions 12 and 13, and is substituted by hydroxy groups at the 3β and 24-positions, and by an oxo group at position 22.
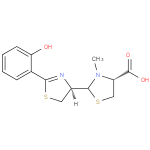
pyochelin (PAMDB110360)
IUPAC:
(4R)- 2-
2- [(4R)-
[(4R)- 2-
2- (2-
(2- hydroxyphenyl)-
hydroxyphenyl)- 4,5-
4,5- dihydro-
dihydro- 1,3-
1,3- thiazol-
thiazol- 4-
4- yl]-
yl]- 3-
3- methyl-
methyl- 1,3-
1,3- thiazolidine-
thiazolidine- 4-
4- carboxylic acid
carboxylic acid
CAS: Not Available
Description: A member of the class of thiazolidines that is (4R)-3-methyl-1,3-thiazolidine-4-carboxylic acid which is substituted at position 2 by a (4R)-2-(2-hydroxyphenyl)-4,5-dihydro-1,3-thiazol-4-yl group. A siderophore, it is it is produced by Pseudomonas aeruginosa (via condensation of salicylic acid and two molecules of cysteine) as a mixture of two easily interconvertible diastereoisomers, pyochelin I (major) and pyochelin II (minor). The enantiomeric compounds, enant-pyochelin, are produced by Pseudomonas fluorescens.
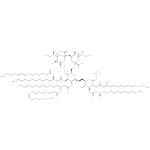
Kdo2-(palmitoleoyl)-lipid IVA (PAMDB110362)
IUPAC:
Not Available
CAS: Not Available
Description: A lipid A oxoanion obtained via deprotonation of the carboxy and phosphate OH groups of (KDO)2-(palmitoleoyl)-lipid IVA; major species at pH 7.3.
(Kdo)2-lipid A, cold adapted (PAMDB110363)
IUPAC:
Not Available
CAS: Not Available
Description: A lipid A oxoanion obtained via deprotonation of the carboxy and phosphate OH groups of (KDO)2-(palmitoleoyl-myristoyl)-lipid A; major species at pH 7.3.
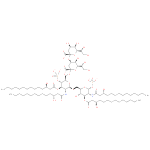
α-Kdo-(2->4)-α-Kdo-(2->6)-lipid IVA (PAMDB110364)
IUPAC:
Not Available
CAS: Not Available
Description: (KDO)2-lipid IVA deprotonated at both phosphono groups and at the uronic acid carboxy groups. It is the major species at pH 7.3.
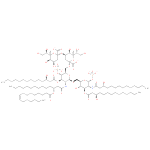
α-D-Kdo-(2→4)-α-D-Kdo-(2→6)-lipid A (PAMDB110365)
IUPAC:
3- deoxy-
deoxy- α-
α- D-
D- manno-
manno- oct-
oct- 2-
2- ulopyranonosyl-
ulopyranonosyl- (2→4)-
(2→4)- 3-
3- deoxy-
deoxy- α-
α- D-
D- manno-
manno- oct-
oct- 2-
2- ulopyranonosyl-
ulopyranonosyl- (2→6)-
(2→6)- 2-
2- deoxy-
deoxy- 2-
2- [(3R)-
[(3R)- 3-
3- (dodecanoyloxy)tetradecanamido]-
(dodecanoyloxy)tetradecanamido]- 4-
4- O-
O- phosphonato-
phosphonato- 3-
3- O-
O- [(3R)-
[(3R)- 3-
3- (tetradecanoyloxy)tetradecanoyl]-
(tetradecanoyloxy)tetradecanoyl]- β-
β- D-
D- glucopyranosyl-
glucopyranosyl- (1→6)-
(1→6)- 2-
2- deoxy-
deoxy- 3-
3- O-
O- [(3R)-
[(3R)- 3-
3- hydroxytetradecanoyl]-
hydroxytetradecanoyl]- 2-
2- [(3R)-
[(3R)- 3-
3- hydroxytetradecanamido]-
hydroxytetradecanamido]- 1-
1- O-
O- phosphonato-
phosphonato- α-
α- D-
D- glucopyranose
glucopyranose
CAS: Not Available
Description: A carbohydrate acid derivative anion that is the hexa-anion of Kdo2-lipid A arising from deprotonation of both the carboxyl and phosphate functions.
α-Kdo-(2->4)-α-Kdo-(2->6)-(lauroyl)-lipid IVA (PAMDB110366)
IUPAC:
Not Available
CAS: Not Available
Description: A lipid A oxoanion obtained via deprotonation of the carboxy and phosphate OH groups of (KDO)2-(lauroyl)-lipid IVA; major species at pH 7.3.