|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110358 |
|---|
|
Identification |
|---|
| Name: |
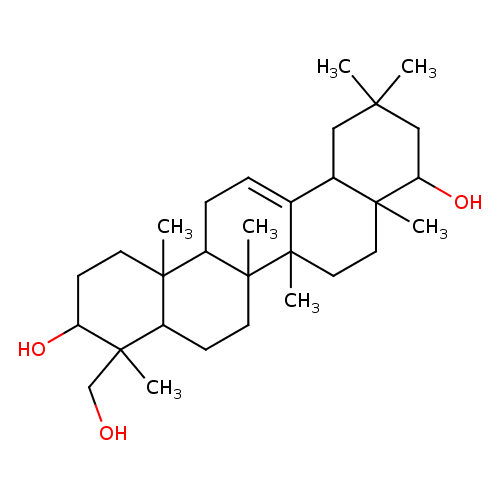
soyasapogenol B |
|---|
| Description: | A pentacyclic triterpenoid that is oleanane containing a double bond between positions 12 and 13 and substituted by hydroxy groups at the 3β, 22β and 24-positions. |
|---|
|
Structure |
|
|---|
| Synonyms: | |
|---|
|
Chemical Formula: |
C30H50O3
|
|---|
| Average Molecular Weight: |
458.72 |
|---|
| Monoisotopic Molecular
Weight: |
458.3759954713 |
|---|
| InChI Key: |
YOQAQNKGFOLRGT-FNBSVUNXSA-N |
|---|
| InChI: |
InChI=1S/C30H50O3/c1-25(2)16-20-19-8-9-22-27(4)12-11-23(32)28(5,18-31)21(27)10-13-30(22,7)29(19,6)15-14-26(20,3)24(33)17-25/h8,20-24,31-33H,9-18H2,1-7H3/t20?,21?,22?,23-,24+,26+,27-,28+,29+,30+/m0/s1 |
|---|
| CAS
number: |
595-15-3 |
|---|
| IUPAC Name: | (3β,22β)-olean-12-ene-3,22,24-triol |
|---|
|
Traditional IUPAC Name: |
4-(hydroxymethyl)-4,6a,6b,8a,11,11,14b-heptamethyl-1,2,3,4a,5,6,7,8,9,10,12,12a,14,14a-tetradecahydropicene-3,9-diol |
|---|
| SMILES: | CC3(CC4(C2(=CCC5(C1(CCC(C(C1CCC(C2(CCC(C(C3)O)4C)C)5C)(CO)C)O)C))))C |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as oleanane triterpenoids. These are triterpenoids with a structure based on the oleanane skeleton, an 4,4,6a,8a,11,14b-heptamethyl-hexadecahydropicene derivative. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Prenol lipids |
|---|
| Sub Class | Triterpenoids |
|---|
|
Direct Parent |
Oleanane triterpenoids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Oleanane triterpenoid
- Steroid
- Cyclic alcohol
- Secondary alcohol
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework |
Aliphatic homopolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
258 - 259 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 258 - 259 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kurosawa Y, Takahara H, Shiraiwa M (2002)UDP-glucuronic acid:soyasapogenol glucuronosyltransferase involved in saponin biosynthesis in germinating soybean seeds. Planta 215, Pubmed: 12172845
- Shibuya M, Hoshino M, Katsube Y, Hayashi H, Kushiro T, Ebizuka Y (2006)Identification of beta-amyrin and sophoradiol 24-hydroxylase by expressed sequence tag mining and functional expression assay. The FEBS journal 273, Pubmed: 16478469
- Evidente A, Cimmino A, Fernández-Aparicio M, Rubiales D, Andolfi A, Melck D (2011)Soyasapogenol B and trans-22-dehydrocam- pesterol from common vetch (Vicia sativa L.) root exudates stimulate broomrape seed germination. Pest management science 67, Pubmed: 21480462
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|