|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110357 |
|---|
|
Identification |
|---|
| Name: |
hederagenin |
|---|
| Description: | A sapogenin that is olean-12-en-28-oic acid substituted by hydroxy groups at positions 3 and 23 (the 3β stereoisomer). |
|---|
|
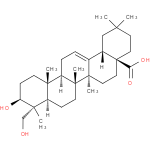
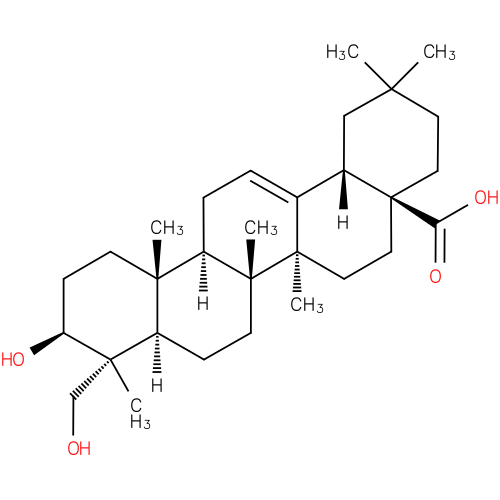
Structure |
|
|---|
| Synonyms: | -
Caulosapogenin
-
Astrantiagenin E
-
Hederagenic acid
-
(3-beta,4-alpha)-3,23-Dihydroxyolean-12-en-28-oic acid
|
|---|
|
Chemical Formula: |
C30H47O4
|
|---|
| Average Molecular Weight: |
471.7 |
|---|
| Monoisotopic Molecular
Weight: |
472.3552600292 |
|---|
| InChI Key: |
PGOYMURMZNDHNS-AFDXFISUSA-M |
|---|
| InChI: |
InChI=1S/C30H48O4/c1-25(2)13-15-30(24(33)34)16-14-28(5)19(20(30)17-25)7-8-22-26(3)11-10-23(32)27(4,18-31)21(26)9-12-29(22,28)6/h7,20-23,31-32H,8-18H2,1-6H3,(H,33,34)/p-1/t20?,21?,22?,23-,26-,27+,28+,29+,30-/m0/s1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | (3β)-3,23-dihydroxyolean-12-en-28-oic acid |
|---|
|
Traditional IUPAC Name: |
Not Available |
|---|
| SMILES: | CC5(CCC4(CCC1(C(=CCC2(C1(CCC3(C2(CCC(C3(C)CO)O)C))C))C4C5)C)C(=O)[O-])C |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as triterpenoids. These are terpene molecules containing six isoprene units. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Prenol lipids |
|---|
| Sub Class | Triterpenoids |
|---|
|
Direct Parent |
Triterpenoids |
|---|
| Alternative Parents |
Not Available |
|---|
| Substituents |
- Triterpenoid
- Steroid
- Cyclic alcohol
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Alcohol
- Carbonyl group
- Primary alcohol
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Organic anion
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework |
Aliphatic homopolycyclic compounds |
|---|
| External Descriptors |
- a triterpenoid (CPD-9481)
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
Not Available |
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
Not Available |
|---|
|
References |
|---|
| References: |
- Jin ZL, Gao N, Zhou D, Chi MG, Yang XM, Xu JP (2012)The extracts of Fructus Akebiae, a preparation containing 90% of the active ingredient hederagenin: serotonin, norepinephrine and dopamine reuptake inhibitor. Pharmacology, biochemistry, and behavior 100, Pubmed: 22005599
- Lorent J, Le Duff CS, Quetin-Leclercq J, Mingeot-Leclercq MP (2013)Induction of highly curved structures in relation to membrane permeabilization and budding by the triterpenoid saponins, a- and d-Hederin. The Journal of biological chemistry 288, Pubmed: 23530040
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|