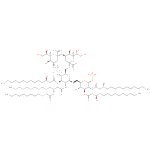
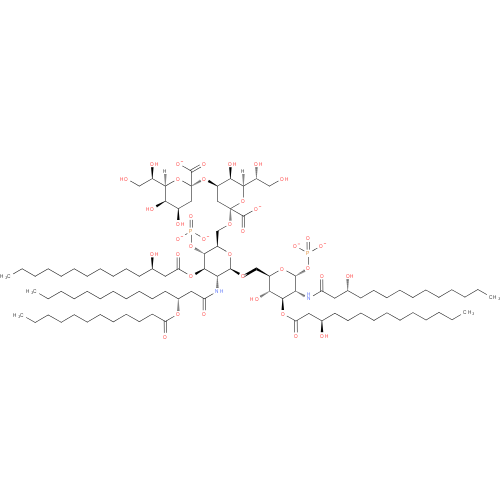
α-Kdo-(2->4)-α-Kdo-(2->6)-(lauroyl)-lipid IVA (PAMDB110366)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB110366 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | α-Kdo-(2->4)-α-Kdo-(2->6)-(lauroyl)-lipid IVA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | A lipid A oxoanion obtained via deprotonation of the carboxy and phosphate OH groups of (KDO)2-(lauroyl)-lipid IVA; major species at pH 7.3. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C96H170N2O38P2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 2022.3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 2027.1376323198 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | JVUUYJGQIVCMIU-UKBUPMDXSA-H | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C96H176N2O38P2/c1-6-11-16-21-26-31-36-41-46-51-66(101)56-76(107)97-81-89(130-79(110)57-67(102)52-47-42-37-32-27-22-17-12-7-2)85(114)74(128-92(81)136-138(122,123)124)64-125-91-82(98-77(108)59-69(54-49-44-39-34-29-24-19-14-9-4)127-78(109)55-50-45-40-35-30-25-20-15-10-5)90(131-80(111)58-68(103)53-48-43-38-33-28-23-18-13-8-3)88(135-137(119,120)121)75(129-91)65-126-95(93(115)116)61-73(84(113)87(133-95)72(106)63-100)132-96(94(117)118)60-70(104)83(112)86(134-96)71(105)62-99/h66-75,81-92,99-106,112-114H,6-65H2,1-5H3,(H,97,107)(H,98,108)(H,115,116)(H,117,118)(H2,119,120,121)(H2,122,123,124)/p-6/t66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,95+,96-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CCCCCCCCCCCC(O)CC(=O)NC1(C(OP([O-])([O-])=O)OC(C(O)C(OC(CC(O)CCCCCCCCCCC)=O)1)COC2(C(NC(CC(OC(CCCCCCCCCCC)=O)CCCCCCCCCCC)=O)C(OC(CC(O)CCCCCCCCCCC)=O)C(C(O2)COC4(C([O-])=O)(O[CH](C(CO)O)C(O)C(OC3(C([O-])=O)(O[CH](C(CO)O)C(O)C(O)C3))C4))OP([O-])([O-])=O)) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as acylaminosugars. These are organic compounds containing a sugar linked to a chain through N-acyl group. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic oxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Organooxygen compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Carbohydrates and carbohydrate conjugates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Acylaminosugars | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||