Search Results for compounds
Searching compounds for
returned 4373 results.
Cyanide (PAMDB000438)
IUPAC:
iminomethanide
CAS: 57-12-5
Description: A cyanide is any chemical compound that contains the cyano group (CN). The metabolism of cyanide in bacteria involves a dioxygenase enzyme that converted cyanide directly to ammonia, without the formation of cyanate.
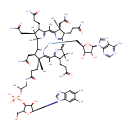
Adenosylcobalamin (PAMDB000439)
IUPAC:
{[(2S,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl}[(2R,3S,4S,8S,9S,14S,18R,19R)-4,9,14-tris(2-carbamoylethyl)-3,8,19-tris(carbamoylmethyl)-18-(2-{[(2R)-2-[({[(2R,3S,4R,5S)-5-(5,6-dimethyl-1H-1,3-benzodiazol-1-yl)-4-hydroxy-2-(hydroxymethyl)oxolan-3-yl]oxy}(hydroxy)phosphoryl)oxy]propyl]carbamoyl}ethyl)-2,3,6,8,13,13,16,18-octamethyl-20,21,22,23-tetraazapentacyclo[15.2.1.1?,??1?????1??,???tricosa-5(23),6,10(22),11,15(21),16-hexaen-20-yl]cobaltylium
CAS: 13870-90-1
Description: Adenosylcobalamin is one of two metabolically active forms of vitamin B12. it acts as a coenzyme in the reaction catalyzed by methylmalonyl-CoA mutase. A cobalamin (cbl) derivative in which the substituent is deoxyadenosyl. (E.C. 5.4.99.2).
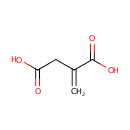
Itaconic acid (PAMDB000440)
IUPAC:
2-methylidenebutanedioic acid
CAS: 97-65-4
Description: Itaconic acid is an intermediate in the C5-Branched dibasic acid metabolism, a substrate for the enzyme Succinate-CoA ligase (ADP-forming) (EC:6.2.1.5)(Kegg)
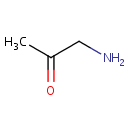
Aminoacetone (PAMDB000441)
IUPAC:
1-aminopropan-2-one
CAS: 298-08-8
Description: Threonine dehydrogenase catalyzes the oxidation of threonine by NAD+ to glycine and acetyl-CoA (5), but when the ratio acetyl-CoA/CoA increases in nutritional deprivation (e.g., in diabetes) the enzyme produces AA. (Chem. Res. Toxicol., 14 (9), 1323 -1329, 2001);
Coproporphyrinogen I (PAMDB000442)
IUPAC:
3-[9,14,19-tris(2-carboxyethyl)-5,10,15,20-tetramethyl-21,22,23,24-tetraazapentacyclo[16.2.1.1?,??1????.1??,???tetracosa-1(20),3,5,8,10,13,15,18-octaen-4-yl]propanoic acid
CAS: 31110-56-2
Description: Coproporphyrinogen is formed by Uroporphyrinogen decarboxylase from Uroporphyrinogen by decarboxylation of 4 acetates.
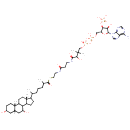
3alpha,7alpha-Dihydroxy-5beta-cholestanoyl-CoA (PAMDB000443)
IUPAC:
{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-2-[({[({3-[(2-{[2-({6-[(2S,5R,7S,9R,15R)-5,9-dihydroxy-2,15-dimethyltetracyclo[8.7.0.0?,??0??,???heptadecan-14-yl]-2-methylheptanoyl}sulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]-3-hydroxy-2,2-dimethylpropoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)methyl]-4-hydroxyoxolan-3-yl]oxy}phosphonic acid
CAS: 2461-62-3
Description: Trans-octadec-2-enoyl-CoA is a member of the chemical class known as Coenzyme A and Derivatives. These are derivative of vitamin B5 containing a 4'-phosphopantetheine moiety attached to a diphospho-adenosine.
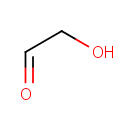
Glycolaldehyde (PAMDB000444)
IUPAC:
2-hydroxyacetaldehyde
CAS: 141-46-8
Description: Glycolaldehyde (HOCH2-CH=O, IUPAC name 2-hydroxyethanal) is a type of diose (2-carbon monosaccharide). Glycolaldehyde is readily converted to acetyl coenzyme A. It has an aldehyde and a hydroxyl group. However, it is not actually a sugar, because there is only one hydroxyl group. Glycolaldehyde is formed from many sources, including the amino acid glycine and from purone catabolism. It can form by action of ketolase on fructose 1,6-bisphosphate in an alternate glycolysis pathway. This compound is transferred by thiamin pyrophosphate during the pentose phosphate shunt.
(S)-b-aminoisobutyric acid (PAMDB000445)
IUPAC:
(2S)-3-amino-2-methylpropanoic acid
CAS: 4249-19-8
Description: Beta-Aminoisobutyric acid is a non-protein amino acid originating from the catabolism of thymine and valine. Beta-Aminoisobutyric acid occurs in two isomeric forms. The S-enantiomer of beta-Aminoisobutyric acid is predominantly derived from the catabolism of valine. It has been suggested that an altered homoeostasis of b-alanine underlies some of the abnormalities with a dihydropyrimidine dehydrogenase (DPD). DPD constitutes the first step of the pyrimidine degradation pathway, in which the pyrimidine bases uracil and thymine are catabolized to b-alanine and the R-enantiomer of beta-Aminoisobutyric acid respectively. In normal situation with an intact pyrimidine degradation pathway, R-methylmalonic acid semialdehyde can be synthesized directly from the catabolism of thymine. Hence, there might be less cross-over between the valine and thymine pathway, allowing the conversion of S-methylmalonic acid semialdehyde into S-beta-Aminoisobutyric acid and the subsequent accumulation of S-beta-Aminoisobutyric acid. (PMID: 14705962, 14292857, 14453202)
Molybdopterin (PAMDB000446)
IUPAC:
({4-hydroxy-2-imino-6,7-disulfanyl-1H,2H,5H,5aH,8H,9aH,10H-pyrano[3,2-g]pteridin-8-yl}methoxy)phosphonic acid
CAS: 73508-07-3
Description: Molybdopterins are a class of biochemical cofactor that are used in many different enzymes. The simplest structure of molybdopterin contains a pyranopterin coordinated to molybdenum. The pyranopterin structure is a fused ring system containing a pyran fused to pterin. In addition, the pyran ring is substituted with two thiols and an alkyl phosphate. In molybdopterin, the thiols coordinate to molybdenum. In some cases, the alkyl phosphate group is replaced by an alkyl diphosphate nucleotide. -- Wikipedia
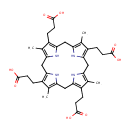
Uroporphyrinogen I (PAMDB000447)
IUPAC:
3-[9,14,19-tris(2-carboxyethyl)-5,10,15,20-tetrakis(carboxymethyl)-21,22,23,24-tetraazapentacyclo[16.2.1.1?,??1????.1??,???tetracosa-1(20),3,5,8,10,13,15,18-octaen-4-yl]propanoic acid
CAS: 1867-62-5
Description: Uroporphyrinogens are porphyrinogen variants in which each pyrrole ring has one acetate side chain and one propionate side chain; it is formed by condensation 4 four molecules of porphobilinogen. 4 isomers are possible but only 2 commoly are found, types and I. Uroporphyrinogen I is a functional intermediate in heme biosynthesis while Uroporphyrinogen is produced in an abortive side reaction.