|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000447 |
|---|
|
Identification |
|---|
| Name: |
Uroporphyrinogen I |
|---|
| Description: | Uroporphyrinogens are porphyrinogen variants in which each pyrrole ring has one acetate side chain and one propionate side chain; it is formed by condensation 4 four molecules of porphobilinogen. 4 isomers are possible but only 2 commoly are found, types and I. Uroporphyrinogen I is a functional intermediate in heme biosynthesis while Uroporphyrinogen is produced in an abortive side reaction. |
|---|
|
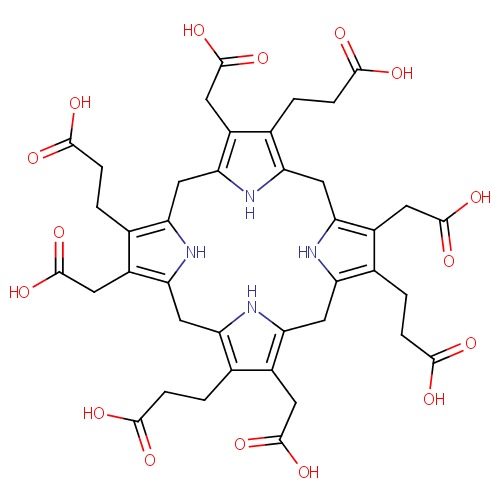
Structure |
|
|---|
| Synonyms: | - 3,8,13,18-Tetrakis(carboxymethyl)-5,10,15,20,22,24-hexahydro-(8CI)-2,7,12,17-Porphinetetrapropionate
- 3,8,13,18-Tetrakis(carboxymethyl)-5,10,15,20,22,24-hexahydro-(8CI)-2,7,12,17-Porphinetetrapropionic acid
- 3,8,13,18-Tetrakis(carboxymethyl)-5,10,15,20,22,24-hexahydro-21H,23H-Porphine-2,7,12,17-tetrapropanoate
- 3,8,13,18-Tetrakis(carboxymethyl)-5,10,15,20,22,24-hexahydro-21H,23H-Porphine-2,7,12,17-tetrapropanoic acid
- 3,8,13,18-Tetrakis(carboxymethyl)-5,10,15,20,22,24-hexahydroporphyrin-2,7,12,17-tetrapropanoate
- 3,8,13,18-Tetrakis(carboxymethyl)-5,10,15,20,22,24-hexahydroporphyrin-2,7,12,17-tetrapropanoic acid
- Uroporphyrinogen I
- Uroporphyrinogen III
|
|---|
|
Chemical Formula: |
C40H44N4O16 |
|---|
| Average Molecular Weight: |
836.7946 |
|---|
| Monoisotopic Molecular
Weight: |
836.27523138 |
|---|
| InChI Key: |
QTTNOSKSLATGQB-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C40H44N4O16/c45-33(46)5-1-17-21(9-37(53)54)29-14-26-19(3-7-35(49)50)23(11-39(57)58)31(43-26)16-28-20(4-8-36(51)52)24(12-40(59)60)32(44-28)15-27-18(2-6-34(47)48)22(10-38(55)56)30(42-27)13-25(17)41-29/h41-44H,1-16H2,(H,45,46)(H,47,48)(H,49,50)(H,51,52)(H,53,54)(H,55,56)(H,57,58)(H,59,60) |
|---|
| CAS
number: |
1867-62-5 |
|---|
| IUPAC Name: | 3-[9,14,19-tris(2-carboxyethyl)-5,10,15,20-tetrakis(carboxymethyl)-21,22,23,24-tetraazapentacyclo[16.2.1.1?,??1????.1??,???tetracosa-1(20),3,5,8,10,13,15,18-octaen-4-yl]propanoic acid |
|---|
|
Traditional IUPAC Name: |
uroporphyrinogen I |
|---|
| SMILES: | OC(=O)CCC1=C2CC3=C(CC(O)=O)C(CCC(O)=O)=C(CC4=C(CC(O)=O)C(CCC(O)=O)=C(CC5=C(CC(O)=O)C(CCC(O)=O)=C(CC(N2)=C1CC(O)=O)N5)N4)N3 |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as porphyrins. These are compounds containing a fundamental skeleton of four pyrrole nuclei united through the alpha-positions by four methine groups to form a macrocyclic structure. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
|
Class |
Tetrapyrroles and derivatives |
|---|
| Sub Class | Porphyrins |
|---|
|
Direct Parent |
Porphyrins |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Porphyrin
- Substituted pyrrole
- Heteroaromatic compound
- Pyrrole
- Azacycle
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -8 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
- Porphyrin and chlorophyll metabolism pae00860
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Maines MD, Mayer RD: Inhibition of testicular cytochrome P-450-dependent steroid biosynthesis by cis-platinum. Reversal by human chorionic gonadotropin. J Biol Chem. 1985 May 25;260(10):6063-8. Pubmed: 4039724
- Mukerji SK, Pimstone NR: Defective human erythrocyte uroporphyrinogen decarboxylase in familial porphyria cutanea tarda: the metabolic lesion or the result of endogenous porphyrinemia? Biochem Biophys Res Commun. 1988 Jul 15;154(1):39-46. Pubmed: 3395340
|
|---|
| Synthesis Reference: |
Burton, Gerardo; Fagerness, Paul E.; Hosozawa, Shigeki; Jordan, Peter M.; Scott, A. Ian. Carbon-13 NMR evidence for a new intermediate, pre-uroporphyrinogen, in the enzymic transformation of porphobilinogen into uroporphyrinogens I and III. Journal of |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|