Search Results for compounds
Searching compounds for
returned 4373 results.
Displaying compounds 3101 - 3110 of
4373 in total
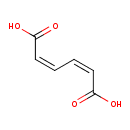
cis,cis-muconate (PAMDB110036)
IUPAC:
(2Z,4Z)-hexa-2,4-dienedioate
CAS: 1119-72-8
Description: A muconate that is the conjugate base of (2Z,4Z)-5-carboxypenta-2,4-dienoate.
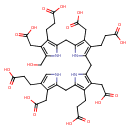
preuroporphyrinogen (PAMDB110037)
IUPAC:
3,8,13,18-tetrakis(carboxylatomethyl)-19-(hydroxymethyl)bilane-2,7,12,17-tetrapropanoate
CAS: 73023-76-4
Description: Octaanion of preuroporphyrinogen arising from global deprotonation of the eight carboxy groups; major species at pH 7.3.
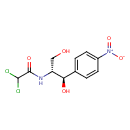
chloramphenicol (PAMDB110038)
IUPAC:
2,2-dichloro-N-[(1R,2R)-1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl]acetamide
CAS: 56-75-7
Description: An antibiotic first isolated from cultures of Streptomyces venequelae in 1947 but now produced synthetically. It has a relatively simple structure and was the first broad-spectrum antibiotic to be discovered. It acts by interfering with bacterial protein synthesis and is mainly bacteriostatic. (From Martindale, The Extra Pharmacopoeia, 29th ed, p106)
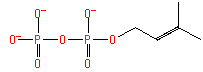
dimethylallyl diphosphate (PAMDB110041)
IUPAC:
Not Available
CAS: Not Available
Description: Not Available
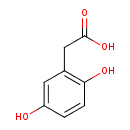
homogentisate (PAMDB110042)
IUPAC:
(2,5-dihydroxyphenyl)acetate
CAS: 451-13-8
Description: A dihydroxy monocarboxylic acid anion that is the conjugate base of (2,6-dihydroxyphenyl)acetic (homogentisic) acid, arising from deprotonation of the carboxy group.
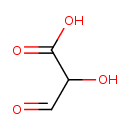
tartronate semialdehyde (PAMDB110045)
IUPAC:
2-hydroxy-3-oxopropanoate
CAS: Not Available
Description: The conjugate base of 2-hydroxy-3-oxopropanoic acid; major species at pH 7.3.
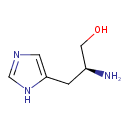
histidinol (PAMDB110046)
IUPAC:
(2S)-2-amino-3-(1H-imidazol-4-yl)propan-1-ol
CAS: 4836-52-6
Description: An amino alcohol that is propanol substituted by 1H-imidazol-4-yl group at position 3 and an amino group at position 2 (the 2S stereoisomer).
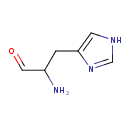
histidinal (PAMDB110047)
IUPAC:
(2S)-1-(1H-imidazol-4-yl)-3-oxopropan-2-aminium
CAS: 23784-15-8
Description: An organic cation that is the conjugate acid of L-histidinal, arising from protonation of the amino group; major species at pH 7.3.
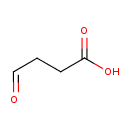
succinate semialdehyde (PAMDB110048)
IUPAC:
4-oxobutanoate
CAS: 692-29-5
Description: The conjugate base of 4-oxobutanoic acid; major species at pH 7.3.