|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110042 |
|---|
|
Identification |
|---|
| Name: |
homogentisate |
|---|
| Description: | A dihydroxy monocarboxylic acid anion that is the conjugate base of (2,6-dihydroxyphenyl)acetic (homogentisic) acid, arising from deprotonation of the carboxy group. |
|---|
|
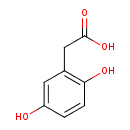
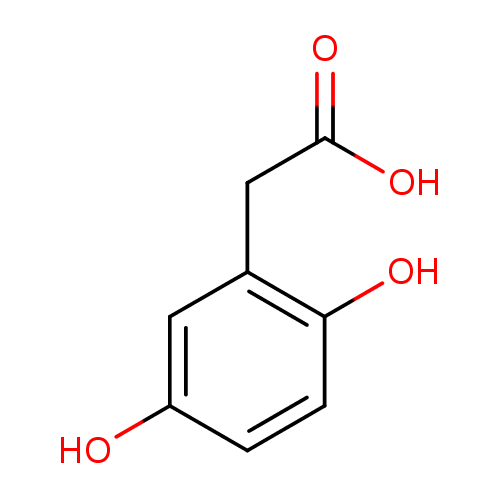
Structure |
|
|---|
| Synonyms: | -
2,5-dihydroxyphenylacetate
|
|---|
|
Chemical Formula: |
C8H7O4
|
|---|
| Average Molecular Weight: |
167.14 |
|---|
| Monoisotopic Molecular
Weight: |
168.0422587452 |
|---|
| InChI Key: |
IGMNYECMUMZDDF-UHFFFAOYSA-M |
|---|
| InChI: |
InChI=1S/C8H8O4/c9-6-1-2-7(10)5(3-6)4-8(11)12/h1-3,9-10H,4H2,(H,11,12)/p-1 |
|---|
| CAS
number: |
451-13-8 |
|---|
| IUPAC Name: | (2,5-dihydroxyphenyl)acetate |
|---|
|
Traditional IUPAC Name: |
homogentisic acid |
|---|
| SMILES: | C1(C(=CC(=C(C=1)O)CC([O-])=O)O) |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as 2(hydroxyphenyl)acetic acids. These are phenylacetic acids that carry a hydroxyl group at the 2-position. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Benzenoids |
|---|
| Sub Class | Benzene and substituted derivatives |
|---|
|
Direct Parent |
2(hydroxyphenyl)acetic acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- 2(hydroxyphenyl)acetic acid
- Hydroquinone
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
153 °C |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | 153 °C | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | 850 mg/mL at 25 °C | Not Available | | LogP | 0.86 | SANGSTER (1994) |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
| Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Pegasus III TOF-MS system, Leco; GC 6890, Agilent Technologies) | splash10-000t-0945000000-2f4b9ef9c9ee6e7cd1ed | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (3 TMS) | splash10-0016-3955000000-1cdc64ab3032ff29df29 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-00di-0900000000-9c395aa569b4b54f8681 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-00xs-9500000000-ab92286f0d5bc843814b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-014l-9000000000-35740a40f4fdf3e84c3c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-014i-0900000000-f4eba60d63bd99e18b8b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-00di-0900000000-12e330dc18f0b128b961 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-00di-0900000000-4bd53b0472edd31e8609 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-00di-0900000000-7121173193a9ba3751a4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-00di-1900000000-04eca21bf25c671b8b6e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Negative | splash10-00di-0900000000-780d3740762c2ff89d9a | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available |
|---|
|
|---|
|
References |
|---|
| References: |
- Shoemaker JD, Elliott WH: Automated screening of urine samples for carbohydrates, organic and amino acids after treatment with urease. J Chromatogr. 1991 Jan 2;562(1-2):125-38. [2026685 ]
- Phornphutkul C, Introne WJ, Perry MB, Bernardini I, Murphey MD, Fitzpatrick DL, Anderson PD, Huizing M, Anikster Y, Gerber LH, Gahl WA: Natural history of alkaptonuria. N Engl J Med. 2002 Dec 26;347(26):2111-21. [12501223 ]
- La Du BN Jr: Are we ready to try to cure alkaptonuria? Am J Hum Genet. 1998 Apr;62(4):765-7. [9529368 ]
- Concepcion Masip T, Banares Baudet F, Traba ML, Rodriguez de Minon Cifuentes JL: [Alkaptonuria, prostatic calculi, and ectopic ureter] Actas Urol Esp. 1997 Feb;21(2):167-70. [9214216 ]
- Ehongo A, Schrooyen M, Pereleux A: [Important bilateral corneal astigmatism in a case of ocular ochronosis] Bull Soc Belge Ophtalmol. 2005;(295):17-21. [15849984 ]
- Deutsch JC, Santhosh-Kumar CR: Quantitation of homogentisic acid in normal human plasma. J Chromatogr B Biomed Appl. 1996 Feb 23;677(1):147-51. [8925087 ]
- Janocha S, Wolz W, Srsen S, Srsnova K, Montagutelli X, Guenet JL, Grimm T, Kress W, Muller CR: The human gene for alkaptonuria (AKU) maps to chromosome 3q. Genomics. 1994 Jan 1;19(1):5-8. [8188241 ]
- de Haas V, Carbasius Weber EC, de Klerk JB, Bakker HD, Smit GP, Huijbers WA, Duran M, Poll-The BT: The success of dietary protein restriction in alkaptonuria patients is age-dependent. J Inherit Metab Dis. 1998 Dec;21(8):791-8. [9870204 ]
- Hegedus ZL, Nayak U: Homogentisic acid and structurally related compounds as intermediates in plasma soluble melanin formation and in tissue toxicities. Arch Int Physiol Biochim Biophys. 1994 May-Jun;102(3):175-81. [8000039 ]
- Venkataseshan VS, Chandra B, Graziano V, Steinlauf P, Marquet E, Irmiere V, Needle MA: Alkaptonuria and renal failure: a case report and review of the literature. Mod Pathol. 1992 Jul;5(4):464-71. [1495952 ]
- Bory C, Boulieu R, Chantin C, Mathieu M: Diagnosis of alcaptonuria: rapid analysis of homogentisic acid by HPLC. Clin Chim Acta. 1990 Jul;189(1):7-11. [2383921 ]
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Download (PDF) |
|---|
|
Links |
|---|
| External Links: |
|
|---|