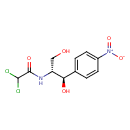
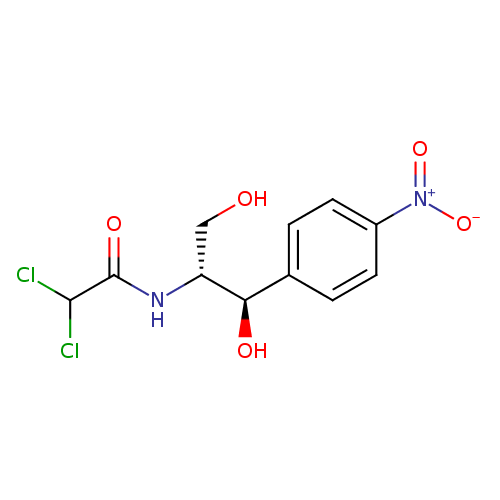
chloramphenicol (PAMDB110038)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB110038 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | chloramphenicol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | An antibiotic first isolated from cultures of Streptomyces venequelae in 1947 but now produced synthetically. It has a relatively simple structure and was the first broad-spectrum antibiotic to be discovered. It acts by interfering with bacterial protein synthesis and is mainly bacteriostatic. (From Martindale, The Extra Pharmacopoeia, 29th ed, p106) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: | • Chloramex • Chloramphenicolum • Chlornitromycin • Chlorocid • Chlorocol • Chloromycetin • Cloramfenicol • D-(-)-2,2-dichloro-N-(beta-Hydroxy-alpha-(hydroxymethyl)-P-nitrophenylethyl)acetamide • D-(-)-threo-1-P-Nitrophenyl-2-dichloroacetylamino-1,3-propanediol • Fenicol • Globenicol • Halomycetin • Laevomycetinum • Levomicetina • Levomycetin • Oleomycetin • Sificetina • D-(-)-2,2-dichloro-N-(b-Hydroxy-a-(hydroxymethyl)-P-nitrophenylethyl)acetamide • D-(-)-2,2-dichloro-N-(?-hydroxy-?-(hydroxymethyl)-P-nitrophenylethyl)acetamide • CAF • CAP • Chloramfenikol • Chloramphenicole • Chloroamphenicol • Cloroamfenicolo • D-Chloramphenicol • Detreomycin • Kloramfenikol • Cloranfenicol • Ophthochlor • Syntomycin | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C11H12N2O5Cl2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 323.13 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 322.0123269261 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | WIIZWVCIJKGZOK-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C11H12Cl2N2O5/c12-10(13)11(18)14-8(5-16)9(17)6-1-3-7(4-2-6)15(19)20/h1-4,8-10,16-17H,5H2,(H,14,18) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 56-75-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | 2,2-dichloro-N-[(1R,2R)-1,3-dihydroxy-1-(4-nitrophenyl)propan-2-yl]acetamide | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | chloramphenicol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | C(O)C(NC(=O)C(Cl)Cl)C(C1(C=CC(N(=O)=O)=CC=1))O | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as nitrobenzenes. These are compounds containing a nitrobenzene moiety, which consists of a benzene ring with a carbon bearing a nitro group. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Benzenoids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Benzene and substituted derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Nitrobenzenes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Nitrobenzenes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aromatic homomonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | 0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | 150.5 °C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||