|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB110045 |
|---|
|
Identification |
|---|
| Name: |
tartronate semialdehyde |
|---|
| Description: | The conjugate base of 2-hydroxy-3-oxopropanoic acid; major species at pH 7.3. |
|---|
|
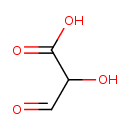
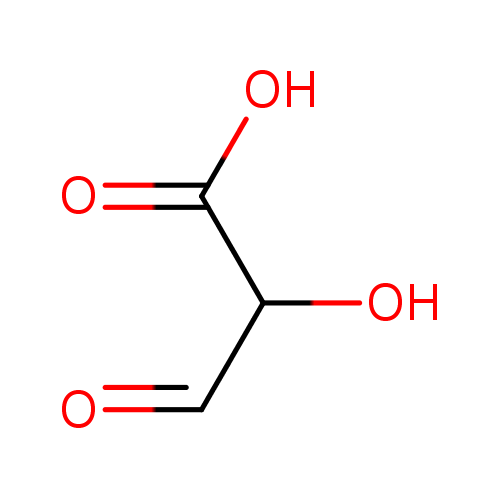
Structure |
|
|---|
| Synonyms: | -
2-hydroxy-3-oxopropanoate
-
tartronic semialdehyde
-
hydroxymalonaldehydic acid
-
tartronate-S-ald
|
|---|
|
Chemical Formula: |
C3H3O4
|
|---|
| Average Molecular Weight: |
103.05 |
|---|
| Monoisotopic Molecular
Weight: |
104.0109586168 |
|---|
| InChI Key: |
QWBAFPFNGRFSFB-UHFFFAOYSA-M |
|---|
| InChI: |
InChI=1S/C3H4O4/c4-1-2(5)3(6)7/h1-2,5H,(H,6,7)/p-1 |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 2-hydroxy-3-oxopropanoate |
|---|
|
Traditional IUPAC Name: |
tartronate semialdehyde |
|---|
| SMILES: | [CH](=O)C(O)C(=O)[O-] |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of chemical entities known as monosaccharides. These are compounds containing one carbohydrate unit not glycosidically linked to another such unit, and no set of two or more glycosidically linked carbohydrate units. Monosaccharides have the general formula CnH2nOn. |
|---|
|
Kingdom |
Chemical entities |
|---|
| Super Class | Organic compounds |
|---|
|
Class |
Organic oxygen compounds |
|---|
| Sub Class | Organooxygen compounds |
|---|
|
Direct Parent |
Monosaccharides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha-hydroxy acid
- Hydroxy acid
- 1,3-dicarbonyl compound
- Monosaccharide
- Alpha-hydroxyaldehyde
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Aldehyde
- Alcohol
- Carbonyl group
- Organic oxide
- Hydrocarbon derivative
- Short-chain aldehyde
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
| Property | Value | Reference |
|---|
| Melting Point | Not Available | Not Available | | Boiling Point | Not Available | Not Available | | Water Solubility | Not Available | Not Available | | LogP | Not Available | Not Available |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Not Available |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|