Search Results for compounds
Searching compounds for
returned 4373 results.
Displaying compounds 1011 - 1020 of
4373 in total
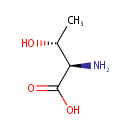
D-Threonine (PAMDB001914)
IUPAC:
(2R,3R)-2-amino-3-hydroxybutanoic acid
CAS: 632-20-2
Description: D-threonine is a member of the chemical class known as Alpha Amino Acids and Derivatives. These are amino acids in which the amino group is attached to the carbon atom immediately adjacent to the carboxylate group (alpha carbon) D-threonine is invovled in D-Amino acids degradation.
Demethylmenaquinone-7 (PAMDB001916)
IUPAC:
2-[(2E,6E,10E,14E,18E,22E)-3,7,11,15,19,23,27-heptamethyloctacosa-2,6,10,14,18,22,26-heptaen-1-yl]-1,4-dihydronaphthalene-1,4-dione
CAS: Not Available
Description: Demethylmenaquinone-7 is a member of the chemical class known as Sesquaterpenes. Menaquinones and demethaquinones are isoprenoid quinones of the naphthalene series, and are constituents of bacterial plasma membranes, where they play important roles in electron transfer and oxidative phosphorylation. Menaquinones and demethaquinones are named MK-n or DMK-n, respectively, where the n refers to the number of prenyl units present in the side chain Demethylmenaquinone-7 is involved in cofactor (menaquinone and ubiquinone) biosynthesis. Demethylquinones are converted to menaquinones via the enzyme ubiE.
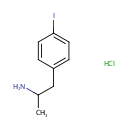
Deoxycytidine 5'-phosphate (PAMDB001921)
IUPAC:
1-(4-iodophenyl)propan-2-amine hydrochloride
CAS: 2498-41-1
Description: Deoxycytidine 5'-phosphate is a nucleoside monophosphate. It is related to the common nucleic acid CTP, or cytidine triphosphate wwith the -OH group on the 2' carbon on the nucleotide's pentose removed (hence the deoxy-part of the name) and with two fewer phosphates.
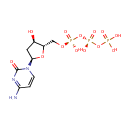
Deoxycytidine 5'-triphosphate (PAMDB001922)
IUPAC:
({[({[(2S,3R,5R)-5-(4-amino-2-oxo-1,2-dihydropyrimidin-1-yl)-3-hydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)phosphonic acid
CAS: 2056-98-6
Description: Deoxycytidine triphosphate (dCTP) is a cytidine nucleotide triphosphate that is used whenever DNA is synthesized by DNA polymerase. During DNA synthesis dCTP has the PPi (pyrophosphate) cleaved off and the dCMP is incorporated into the DNA strand at the 3' end.
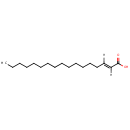
Heptadecenoic acid (PAMDB001933)
IUPAC:
(2E)-heptadec-2-enoic acid
CAS: 26265-99-6
Description: Heptadecenoic acid is a member of the chemical class known as Unsaturated Fatty Acids. These are fatty acids whose chain contains at least one CC double bond. (inferred from compound structure)
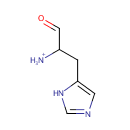
L-Histidinal (PAMDB001944)
IUPAC:
1-(1H-imidazol-5-yl)-3-oxopropan-2-aminium
CAS: Not Available
Description: L-histidinal is a member of the chemical class known as Imidazoles. These are compounds containing an imidazole ring, which is an aromatic five-member ring with two nitrogen atoms at positions 1 and 3, and three carbon atoms. L-histidinal is invovled in Biosynthesis of secondary metabolites, and Histidine metabolism. (KEGG)
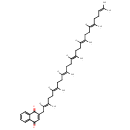
Menaquinol 6 (PAMDB001946)
IUPAC:
2-[(2E,6E,10E,14E,18E)-3,7,11,15,19,23-hexamethyltetracosa-2,6,10,14,18,22-hexaen-1-yl]-3-methylnaphthalene-1,4-diol
CAS: 39776-48-2
Description: Menaquinol 6 is a polyprenylhydroquinone having a hexaprenyl moiety at position 2 and a methyl group at position 3. It is a substrate for Dimethyl sulfoxide reductase (dmsA). This enzyme catalyzes the reduction of dimethyl sulfoxide (DMSO) to dimethyl sulfide (DMS) using the following reaction: Dimethylsulfide + menaquinone + H2O = dimethylsulfoxide + menaquinol. DMSO reductase serves as the terminal reductase under anaerobic conditions, with DMSO being the terminal electron acceptor. Terminal reductase during anaerobic growth on various sulfoxides and N-oxide compounds. This enzyme allows P. aeruginosa to grow anaerobically on DMSO as respiratory oxidant. Menaquinol 6 is generated by Ubiquinone/menaquinone biosynthesis methyltransferase (ubiE). This enzyme is required for the conversion of demethylmenaquinone (DMKH2) to menaquinone (MKH2) and has the following catalytic activity: A demethylmenaquinone + S-adenosyl-L-methionine = a menaquinol + S-adenosyl-L-homocysteine.
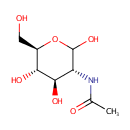
N-Acetylglucosamine (PAMDB001956)
IUPAC:
N-[(3R,4R,5S,6R)-2,4,5-trihydroxy-6-(hydroxymethyl)oxan-3-yl]acetamide
CAS: 7512-17-6
Description: N-Acetyl-D-Glucosamine (N-acetlyglucosamine, GlcNAc) is a monosaccharide derivative of glucose. Chemically it is an amide between glucosamine and acetic acid. It is part of peptidoglycan, a biopolymer in bacterial cell walls, built from alternating units of GlcNAc and N-acetylmuramic acid (MurNAc), cross-linked with oligopeptides at the lactic acid residue of MurNAc. It is a key component of peptidoglycan synthesis. The peptidoglycan synthesis pathway starts at the cytoplasm, where in six steps the peptidoglycan precursor a UDP-N-acetylmuramoyl-pentapeptide is synthesized. This precursor is then attached to the memberane acceptor all-trans-undecaprenyl phosphate, generating a N-acetylmuramoyl-pentapeptide-diphosphoundecaprenol, also known as lipid I. Another transferase then adds UDP-N-acetyl-D-glucosamine, yielding the complete monomeric unit a lipid II, also known as lipid II. This final lipid intermediate is transferred through the membrane. The peptidoglycan monomers are then polymerized on the outside surface by glycosyltransferases, which form the linear glycan chains, and transpeptidases, which catalyze the formation of peptide crosslinks.
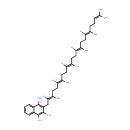
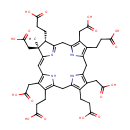
Precorrin-1 (PAMDB001963)
IUPAC:
3-[(4S,5S)-10,14,19-tris(2-carboxyethyl)-5,9,15,20-tetrakis(carboxymethyl)-5-methyl-21,22,23,24-tetraazapentacyclo[16.2.1.1?,??1????.1??,???tetracosa-1(20),3(24),6,8,10,13,15,18-octaen-4-yl]propanoic acid
CAS: Not Available
Description: Precorrin-1 is a member of the chemical class known as Precorrins. These are intermediates formed by methylation at one or more of the four rings prior to the formation of the macrocyclic corrin ring. Precorrin-1 is invovled in Proto- and siroheme biosynthesis.
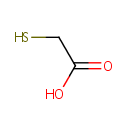
Thioglycolate (PAMDB001967)
IUPAC:
2-sulfanylacetic acid
CAS: 513-66-6
Description: Thioglycolic acid (TGA) is the organic compound HSCH2CO2H. It contains both a thiol (mercaptan) and a carboxylic acid. It is a clear liquid with a strong unpleasant odor. It is readily oxidized by air to the corresponding disulfide [SCH2CO2H]2. It is use during the conversion of chloro-alanine to S-carboxymethyl-L-cysteine.