|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001921 |
|---|
|
Identification |
|---|
| Name: |
Deoxycytidine 5'-phosphate |
|---|
| Description: | Deoxycytidine 5'-phosphate is a nucleoside monophosphate. It is related to the common nucleic acid CTP, or cytidine triphosphate wwith the -OH group on the 2' carbon on the nucleotide's pentose removed (hence the deoxy-part of the name) and with two fewer phosphates. |
|---|
|
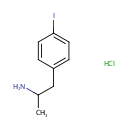
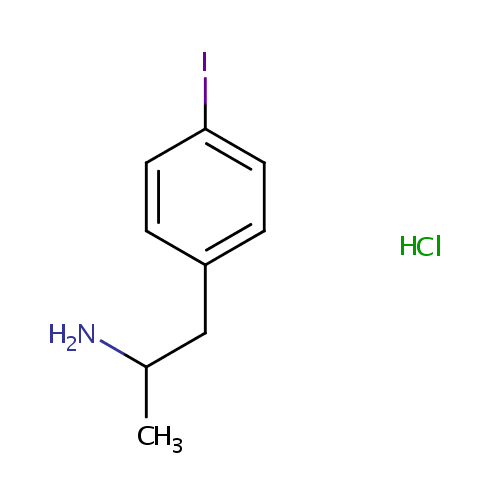
Structure |
|
|---|
| Synonyms: | - 2'-Deoxy-5-methyl-5'-cytidylate
- 2'-Deoxy-5-methyl-5'-cytidylic acid
- 2'-Deoxy-5-methylcytidine 5'-(dihydrogen phosphate)
- 2'-Deoxy-5-methylcytidine 5'-(dihydrogen phosphoric acid)
- 2'-Deoxy-5-methylcytidine 5'-monophosphate
- 2'-Deoxy-5-methylcytidine 5'-monophosphoric acid
- 5-METHYL-2'-DEOXY-CYTIDINE-5'-MONOPHOSPHATE
- 5-METHYL-2'-deoxy-cytidine-5'-monophosphoric acid
- 5-Methyldeoxycytidine 5'-phosphate
- 5-Methyldeoxycytidine 5'-phosphoric acid
- Deoxy-5-methylcytidylate
- Deoxy-5-methylcytidylic acid
- Deoxycytidine 5'-phosphoric acid
|
|---|
|
Chemical Formula: |
C9H13ClIN |
|---|
| Average Molecular Weight: |
297.564 |
|---|
| Monoisotopic Molecular
Weight: |
296.978120548 |
|---|
| InChI Key: |
JJHFCILQSKMLBS-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C9H12IN.ClH/c1-7(11)6-8-2-4-9(10)5-3-8;/h2-5,7H,6,11H2,1H3;1H |
|---|
| CAS
number: |
2498-41-1 |
|---|
| IUPAC Name: | 1-(4-iodophenyl)propan-2-amine hydrochloride |
|---|
|
Traditional IUPAC Name: |
para-iodoamphetamine hydrochloride |
|---|
| SMILES: | Cl.CC(N)CC1=CC=C(I)C=C1 |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as amphetamines and derivatives. These are organic compounds containing or derived from 1-phenylpropan-2-amine. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
|
Class |
Benzene and substituted derivatives |
|---|
| Sub Class | Phenethylamines |
|---|
|
Direct Parent |
Amphetamines and derivatives |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Amphetamine or derivatives
- Phenylpropane
- Aralkylamine
- Iodobenzene
- Halobenzene
- Aryl iodide
- Aryl halide
- Hydrocarbon derivative
- Hydrochloride
- Primary amine
- Organonitrogen compound
- Organoiodide
- Organohalogen compound
- Primary aliphatic amine
- Amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework |
Aromatic homomonocyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | 16764 | | HMDB ID | Not Available | | Pubchem Compound ID | 210911 | | Kegg ID | C03495 | | ChemSpider ID | 182827 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|