|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001933 |
|---|
|
Identification |
|---|
| Name: |
Heptadecenoic acid |
|---|
| Description: | Heptadecenoic acid is a member of the chemical class known as Unsaturated Fatty Acids. These are fatty acids whose chain contains at least one CC double bond. (inferred from compound structure) |
|---|
|
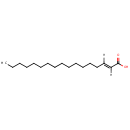
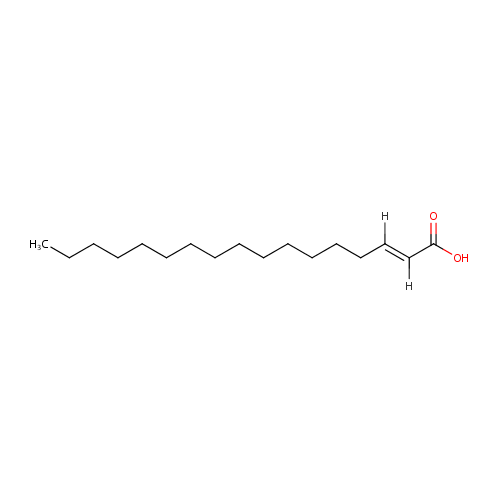
Structure |
|
|---|
| Synonyms: | - (E)-Heptadec-9-enoate
- (E)-Heptadec-9-enoic acid
- 9E-Heptadecenoate
- 9E-Heptadecenoic acid
- Heptadecenoate
|
|---|
|
Chemical Formula: |
C17H32O2 |
|---|
| Average Molecular Weight: |
268.4348 |
|---|
| Monoisotopic Molecular
Weight: |
268.240230268 |
|---|
| InChI Key: |
GEHPRJRWZDWFBJ-FOCLMDBBSA-N |
|---|
| InChI: | InChI=1S/C17H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17(18)19/h15-16H,2-14H2,1H3,(H,18,19)/b16-15+ |
|---|
| CAS
number: |
26265-99-6 |
|---|
| IUPAC Name: | (2E)-heptadec-2-enoic acid |
|---|
|
Traditional IUPAC Name: |
2-heptadecylenic acid |
|---|
| SMILES: | [H]\C(CCCCCCCCCCCCCC)=C(\[H])C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as long-chain fatty acids. These are fatty acids with an aliphatic tail that contains between 13 and 21 carbon atoms. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Fatty Acyls |
|---|
| Sub Class | Fatty acids and conjugates |
|---|
|
Direct Parent |
Long-chain fatty acids |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Long-chain fatty acid
- Unsaturated fatty acid
- Straight chain fatty acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | -1 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Membrane |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Yurtsever D. (2007). Fatty acid methyl ester profiling of Enterococcus and Esherichia coli for microbial source tracking. M.sc. Thesis. Villanova University: U.S.A
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | 5282747 | | Kegg ID | C16536 | | ChemSpider ID | 4445874 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|