|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001956 |
|---|
|
Identification |
|---|
| Name: |
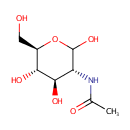
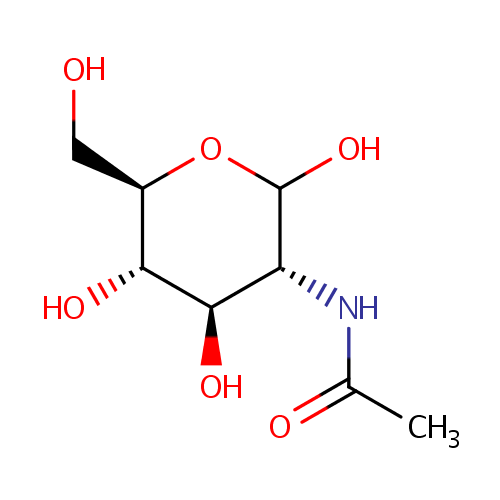
N-Acetylglucosamine |
|---|
| Description: | N-Acetyl-D-Glucosamine (N-acetlyglucosamine, GlcNAc) is a monosaccharide derivative of glucose. Chemically it is an amide between glucosamine and acetic acid. It is part of peptidoglycan, a biopolymer in bacterial cell walls, built from alternating units of GlcNAc and N-acetylmuramic acid (MurNAc), cross-linked with oligopeptides at the lactic acid residue of MurNAc. It is a key component of peptidoglycan synthesis. The peptidoglycan synthesis pathway starts at the cytoplasm, where in six steps the peptidoglycan precursor a UDP-N-acetylmuramoyl-pentapeptide is synthesized. This precursor is then attached to the memberane acceptor all-trans-undecaprenyl phosphate, generating a N-acetylmuramoyl-pentapeptide-diphosphoundecaprenol, also known as lipid I. Another transferase then adds UDP-N-acetyl-D-glucosamine, yielding the complete monomeric unit a lipid II, also known as lipid II. This final lipid intermediate is transferred through the membrane. The peptidoglycan monomers are then polymerized on the outside surface by glycosyltransferases, which form the linear glycan chains, and transpeptidases, which catalyze the formation of peptide crosslinks. |
|---|
|
Structure |
|
|---|
| Synonyms: | - 2-(acetylamino)-2-Deoxy-b-D-glucopyranose
- 2-(Acetylamino)-2-deoxy-beta-D-glucopyranose
- 2-(Acetylamino)-2-deoxy-D-Glucose
- 2-(acetylamino)-2-Deoxy-β-D-glucopyranose
- 2-(Acetylamino)-2-deoxyhexose
- 2-(Acetylamino)-2-deoxyhexose (ACD/Name 4.0)
- 2-acetamido-2-Deoxy-b-D-glucopyranose
- 2-Acetamido-2-deoxy-beta-D-glucopyranose
- 2-Acetamido-2-deoxy-D-glucose
- 2-acetamido-2-Deoxy-β-D-glucopyranose
- 2-Acetamido-2-deoxyglucose
- 2-Acetamido-2-deoxyhexopyranose
- 2-Acetamido-D-glucose
- 2-Acetylamino-2-deoxy-D-glucose
- N-acetylglucosamine
- Acetylglucosamine
- BetaGlcNAc
- D-N-Acetylglucosamine
- Glcnac
- GlcNAc-b
- GlcNAc-beta
- GlcNAc-β
- Glucosamine Complex
- N-Acetyl-b-D-glucosamine
- N-Acetyl-beta-D-glucosamine
- N-Acetyl-D-glucosamine
- N-Acetyl-D-hexosamine
- N-Acetyl-β-D-glucosamine
- N-Acetylchitosamine
- N-Acetylglucosamine
- NAcGlc
- NAG
|
|---|
|
Chemical Formula: |
C8H15NO6 |
|---|
| Average Molecular Weight: |
221.2078 |
|---|
| Monoisotopic Molecular
Weight: |
221.089937217 |
|---|
| InChI Key: |
OVRNDRQMDRJTHS-RTRLPJTCSA-N |
|---|
| InChI: | InChI=1S/C8H15NO6/c1-3(11)9-5-7(13)6(12)4(2-10)15-8(5)14/h4-8,10,12-14H,2H2,1H3,(H,9,11)/t4-,5-,6-,7-,8?/m1/s1 |
|---|
| CAS
number: |
7512-17-6 |
|---|
| IUPAC Name: | N-[(3R,4R,5S,6R)-2,4,5-trihydroxy-6-(hydroxymethyl)oxan-3-yl]acetamide |
|---|
|
Traditional IUPAC Name: |
GlcNAc |
|---|
| SMILES: | CC(=O)N[C@H]1C(O)O[C@H](CO)[C@@H](O)[C@@H]1O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as acylaminosugars. These are organic compounds containing a sugar linked to a chain through N-acyl group. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organooxygen compounds |
|---|
|
Class |
Carbohydrates and carbohydrate conjugates |
|---|
| Sub Class | Aminosaccharides |
|---|
|
Direct Parent |
Acylaminosugars |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Acylaminosugar
- N-acyl-alpha-hexosamine
- Oxane
- Monosaccharide
- Acetamide
- Secondary carboxylic acid amide
- Secondary alcohol
- Polyol
- Hemiacetal
- Carboxamide group
- 1,2-diol
- Oxacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Carboxylic acid amide
- Hydrocarbon derivative
- Primary alcohol
- Organonitrogen compound
- Carbonyl group
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework |
Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
210 °C |
|---|
| Experimental Properties: |
| Property | Value | Source |
|---|
| Water Solubility: | 167 mg/mL | PhysProp |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Collard CD, Montalto MC, Reenstra WR, Buras JA, Stahl GL: Endothelial oxidative stress activates the lectin complement pathway: role of cytokeratin 1. Am J Pathol. 2001 Sep;159(3):1045-54. Pubmed: 11549596
- Hatcher VB, Schwarzmann GO, Jeanloz RW, McArthur JW: Changes in the sialic acid concentration in the major cervical glycoprotein from the bonnet monkey (Macaca radiata) during a hormonally induced cycle. Fertil Steril. 1977 Jun;28(6):682-8. Pubmed: 405259
- Kottgen E, Hell B, Muller C, Kainer F, Tauber R: Developmental changes in the glycosylation and binding properties of human fibronectins. Characterization of the glycan structures and ligand binding of human fibronectins from adult plasma, cord blood and amniotic fluid. Biol Chem Hoppe Seyler. 1989 Dec;370(12):1285-94. Pubmed: 2619923
- Madrid JF, Castells MT, Martinez-Menarguez JA, Aviles M, Hernandez F, Ballesta J: Subcellular characterization of glycoproteins in the principal cells of human gallbladder. A lectin cytochemical study. Histochemistry. 1994 Mar;101(3):195-204. Pubmed: 8056619
- Mollicone R, Candelier JJ, Mennesson B, Couillin P, Venot AP, Oriol R: Five specificity patterns of (1----3)-alpha-L-fucosyltransferase activity defined by use of synthetic oligosaccharide acceptors. Differential expression of the enzymes during human embryonic development and in adult tissues. Carbohydr Res. 1992 Apr 10;228(1):265-76. Pubmed: 1366057
- Nakagawa F, Schulte BA, Spicer SS: Lectin cytochemical evaluation of somatosensory neurons and their peripheral and central processes in rat and man. Cell Tissue Res. 1986;245(3):579-89. Pubmed: 3757018
- Percheron F: [Beta-D-mannosidase] Bull Acad Natl Med. 1995 May;179(5):881-91; discussion 892. Pubmed: 7583460
- Slawson, C., Housley, M. P., Hart, G. W. (2006). "O-GlcNAc cycling: how a single sugar post-translational modification is changing the way we think about signaling networks." J Cell Biochem 97:71-83. Pubmed: 16237703
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. Pubmed: 19212411
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Weiner B, Fischer T, Waxman S: Hemostasis in the era of the chronic anticoagulated patient. J Invasive Cardiol. 2003 Nov;15(11):669-73; quiz 674. Pubmed: 14608143
- Yates AD, Watkins WM: Enzymes involved in the biosynthesis of glycoconjugates. A UDP-2-acetamido-2-deoxy-D-glucose: beta-D-galactopyranosyl-(1 leads to 4)-saccharide (1 leads to 3)-2-acetamido-2-deoxy-beta-D- glucopyranosyltransferase in human serum. Carbohydr Res. 1983 Aug 16;120:251-68. Pubmed: 6226355
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|