|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB001648 |
|---|
|
Identification |
|---|
| Name: |
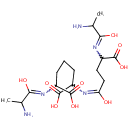
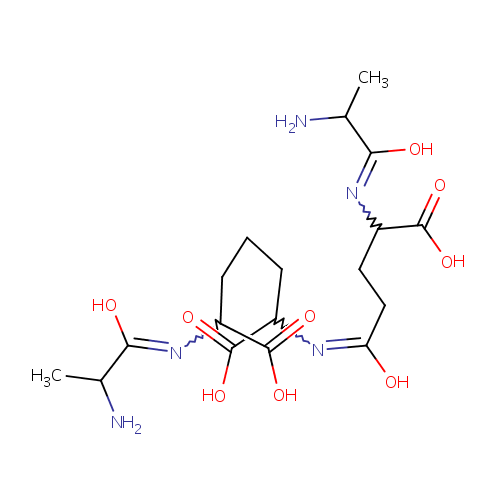
L-alanine-D-glutamate-meso-2,6-diaminoheptanedioate |
|---|
| Description: | L-alanine-d-glutamate-meso-2,6-diaminoheptanedioate belongs to the class of Peptides. These are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another. (inferred from compound structure) |
|---|
|
Structure |
|
|---|
| Synonyms: | - L-Alanine-D-glutamate-meso-2,6-diaminoheptanedioic acid
- L-Alanine-D-glutamic acid-meso-2,6-diaminoheptanedioic acid
|
|---|
|
Chemical Formula: |
C18H31N5O9 |
|---|
| Average Molecular Weight: |
461.4668 |
|---|
| Monoisotopic Molecular
Weight: |
461.212177615 |
|---|
| InChI Key: |
BAPAFFOXTCMVCC-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C18H31N5O9/c1-8(19)14(25)22-11(17(29)30)5-3-4-10(16(27)28)21-13(24)7-6-12(18(31)32)23-15(26)9(2)20/h8-12H,3-7,19-20H2,1-2H3,(H,21,24)(H,22,25)(H,23,26)(H,27,28)(H,29,30)(H,31,32) |
|---|
| CAS
number: |
Not Available |
|---|
| IUPAC Name: | 2-[(2-amino-1-hydroxypropylidene)amino]-6-({4-[(2-amino-1-hydroxypropylidene)amino]-4-carboxy-1-hydroxybutylidene}amino)heptanedioic acid |
|---|
|
Traditional IUPAC Name: |
2-[(2-amino-1-hydroxypropylidene)amino]-6-({4-[(2-amino-1-hydroxypropylidene)amino]-4-carboxy-1-hydroxybutylidene}amino)heptanedioic acid |
|---|
| SMILES: | CC(N)C(O)=NC(CCCC(N=C(O)CCC(N=C(O)C(C)N)C(O)=O)C(O)=O)C(O)=O |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as peptides. These are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
|
Class |
Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
|
Direct Parent |
Peptides |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Alpha peptide
- N-acyl-aliphatic-alpha amino acid
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- Alpha-amino acid or derivatives
- Tricarboxylic acid or derivatives
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboxylic acid
- Carboximidic acid derivative
- Carboximidic acid
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Carbonyl group
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework |
Aliphatic acyclic compounds |
|---|
| External Descriptors |
Not Available |
|---|
|
Physical Properties |
|---|
| State: |
Not Available |
|---|
| Charge: | 0 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | Adenosine triphosphate + Water + L-alanine-D-glutamate-meso-2,6-diaminoheptanedioate > L-alanine-D-glutamate-meso-2,6-diaminoheptanedioate + ADP + Hydrogen ion + PhosphateAdenosine triphosphate + Water + L-alanine-D-glutamate-meso-2,6-diaminoheptanedioate > L-alanine-D-glutamate-meso-2,6-diaminoheptanedioate + ADP + Hydrogen ion + PhosphateN-Acetyl-D-glucosamine(anhydrous)N-Acetylmuramyl-tripeptide + Water > L-alanine-D-glutamate-meso-2,6-diaminoheptanedioate + N-Acetyl-D-glucosamine(anhydrous)N-Acetylmuramic acid1,6-Anhydrous-N-Acetylmuramyl-tripeptide + Water > L-alanine-D-glutamate-meso-2,6-diaminoheptanedioate + 1,6-Anhydro-N-acetylmuramateL-Alanine-D-glutamate-meso-2,6-diaminoheptanedioate-D-alanine + Water > L-alanine-D-glutamate-meso-2,6-diaminoheptanedioate + D-AlanineL-alanine-D-glutamate-meso-2,6-diaminoheptanedioate + Water > Diaminopimelic acid + L-Alanyl-D-glutamateL-alanine-D-glutamate-meso-2,6-diaminoheptanedioate + Adenosine triphosphate + UDP-N-Acetylmuraminate > ADP + Hydrogen ion + Phosphate + UDP-N-Acetylmuramoyl-L-alanyl-D-glutamyl-meso-2,6-diaminoheptanedioate |
|---|
|
Pathways: |
Not Available |
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
Not Available |
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
| Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | 46173126 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|