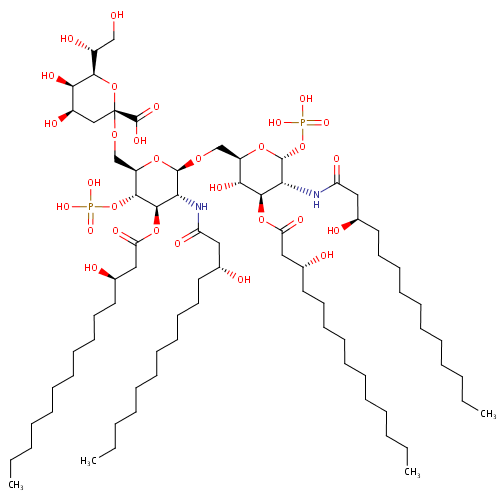
KDO-lipid IV(A) (PAMDB001644)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB001644 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name: | KDO-lipid IV(A) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | KDO-lipid IV(a) is a part of lipopolysaccharide or LPS. Specifically it is lipid IVA glycosylated with a single 3-deoxy-D-manno-octulosonic acid (KDO) residue. KDO-lipid IV(a) is a saccharolipid. The most familiar saccharolipids are the acylated glucosamine precursors of the lipid A component of the lipopolysaccharides in Gram-negative bacteria. Typical lipid A molecules are disaccharides of glucosamine, which are derivatized with as many as seven fatty-acyl chains. The minimal lipopolysaccharide required for growth in Pseudomonas aeruginosa is Kdo2-Lipid A, a hexa-acylated disaccharide of glucosamine that is glycosylated with two 3-deoxy-D-manno-octulosonic acid (Kdo) residues. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C76H142N2O30P2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 1625.8836 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 1624.912264238 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | GPNCBCJEDRRCDW-ACUQGRCXSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C76H142N2O30P2/c1-5-9-13-17-21-25-29-33-37-41-53(80)45-61(86)77-65-71(104-63(88)47-55(82)43-39-35-31-27-23-19-15-11-7-3)68(91)59(102-74(65)108-110(97,98)99)51-100-73-66(78-62(87)46-54(81)42-38-34-30-26-22-18-14-10-6-2)72(105-64(89)48-56(83)44-40-36-32-28-24-20-16-12-8-4)70(107-109(94,95)96)60(103-73)52-101-76(75(92)93)49-57(84)67(90)69(106-76)58(85)50-79/h53-60,65-74,79-85,90-91H,5-52H2,1-4H3,(H,77,86)(H,78,87)(H,92,93)(H2,94,95,96)(H2,97,98,99)/t53-,54-,55-,56-,57-,58-,59-,60-,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,76-/m1/s1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | (2R,4R,5R,6R)-6-[(1R)-1,2-dihydroxyethyl]-4,5-dihydroxy-2-{[(2R,3S,4R,5R,6R)-6-{[(2R,3S,4R,5R,6R)-3-hydroxy-5-[(3R)-3-hydroxytetradecanamido]-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonooxy)oxan-2-yl]methoxy}-5-[(3R)-3-hydroxytetradecanamido]-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-3-(phosphonooxy)oxan-2-yl]methoxy}oxane-2-carboxylic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | (kdo)-lipid iva | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CCCCCCCCCCC[C@@H](O)CC(=O)N[C@H]1[C@@H](OP(O)(O)=O)O[C@H](CO[C@@H]2O[C@H](CO[C@@]3(C[C@@H](O)[C@@H](O)[C@H](O3)[C@H](O)CO)C(O)=O)[C@@H](OP(O)(O)=O)[C@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@H]2NC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](O)[C@@H]1OC(=O)C[C@H](O)CCCCCCCCCCC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of organic compounds known as saccharolipids. These are compounds in which fatty acids are linked directly to a sugar backbone, forming structures that are compatible with membrane bilayers. In the saccharolipids, a sugar substitutes for the glycerol backbone that is present in glycerolipids and glycerophospholipids. The most familiar saccharolipids contain an acylated glucosamine. In contrast to others glycolipids, the fatty acid is not glycosidically linked to the sugar moiety. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Lipids and lipid-like molecules | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Saccharolipids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Saccharolipids | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic heteromonocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Membrane | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | CMP-3-Deoxy-D-manno-octulosonate + 2,3,2'3'-Tetrakis(beta-hydroxymyristoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate > Cytidine monophosphate + Hydrogen ion + KDO-lipid IV(A) CMP-3-Deoxy-D-manno-octulosonate + KDO-lipid IV(A) > Cytidine monophosphate + Hydrogen ion + KDO(2)-lipid IV(A) 2,3,2'3'-Tetrakis(3-hydroxytetradecanoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate + CMP-3-Deoxy-D-manno-octulosonate + 2,3,2'3'-Tetrakis(beta-hydroxymyristoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate <> KDO-lipid IV(A) + Cytidine monophosphate KDO-lipid IV(A) + CMP-3-Deoxy-D-manno-octulosonate <> Di[3-deoxy-D-manno-octulosonyl]-lipid IV(A) + Cytidine monophosphate CMP-3-Deoxy-D-manno-octulosonate + lipid IV<sub>A</sub> <> Hydrogen ion + KDO-lipid IV(A) + Cytidine monophosphate KDO-lipid IV(A) + CMP-3-Deoxy-D-manno-octulosonate <> Hydrogen ion + α-Kdo-(2->4)-α-Kdo-(2->6)-lipid IV<SUB>A</SUB> + Cytidine monophosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in biosynthetic process
- Specific function:
- Essential step in lipopolysaccharides biosynthesis. Acts at transfer of 3-deoxy-D-mono octulonic acid (KDO) from CMP-KDO to a tetraacyldisaccharide 1,4'-bisphosphate precursor of lipid A (lipid IVA). Transfers two molecules of KDO to lipid IVA. Degraded by FtsH; therefore FtsH regulates the addition of the sugar moiety of the LPS and thus the maturation of the LPS precursor
- Gene Name:
- waaA
- Locus Tag:
- PA4988
- Molecular weight:
- 46.5 kDa