Nitrate (PAMDB000468)
| Record Information | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 1/22/2018 11:54:54 AM | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolite ID | PAMDB000468 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | ||||||||||||||||||||||||||||||||||||||||||||||||||||
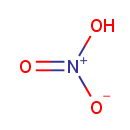
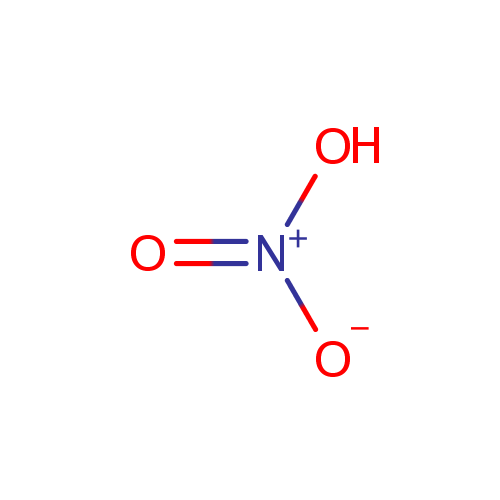
| Name: | Nitrate | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Description: | Nitrate is a salt of nitric acid. In organic chemistry the esters of nitric acid and various alcohols are called nitrates. The nitrate ion is a polyatomic anion with the empirical formula NO3- and a molecular mass of 62.01 daltons; it consists of one central nitrogen atom surrounded by three identical oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a negative one formal charge. Nitrates should not be confused with nitrites, the salts of nitrous acid. Organic compounds containing the nitro functional group (which has the same formula and structure as the nitrate ion save that one of the O2 atoms is replaced by the R group) are known as nitro compounds. Nitrate ions can be toxic. | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | NO3 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Weight: | 62.0049 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Molecular Weight: | 61.987817871 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | NHNBFGGVMKEFGY-UHFFFAOYSA-N | |||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/NO3/c2-1(3)4/q-1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 14797-55-8 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | nitric acid | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | nitric acid | |||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | [O-][N+]([O-])=O | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Taxonomy Description | This compound belongs to the class of inorganic compounds known as non-metal nitrates. These are inorganic non-metallic compoundscontaining a nitrate as its largest oxoanion. | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Inorganic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Homogeneous non-metal compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Non-metal oxoanionic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Non-metal nitrates | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Non-metal nitrates | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Acyclic compounds | |||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 2 Hydrogen ion + Nitrate + Ubiquinol-8 > Water + Nitrite + Ubiquinone-8 +2 Hydrogen ion 2 Hydrogen ion + Menaquinol 8 + Nitrate > Water + Menaquinone 8 + Nitrite +2 Hydrogen ion Ubiquinol-8 + Nitrate > Ubiquinone-8 + Water + Nitrite Menaquinol 8 + Nitrate > Menaquinone 8 + Water + Nitrite NADH + 2 Nitric oxide + 2 Oxygen > Hydrogen ion + NAD +2 Nitrate NADPH + 2 Nitric oxide + 2 Oxygen > Hydrogen ion + NADP +2 Nitrate Nitrite + Acceptor + Water + Acceptor <> Nitrate + Reduced acceptor + Reduced acceptor 2 Ferricytochrome c + Nitrite + Water <> Nitrate +2 Ferrocytochrome c +2 Hydrogen ion Nitrite + Water + Cytochromes-C-Oxidized <> Nitrate + Cytochromes-C-Reduced NAD(P)H + Nitric oxide + Oxygen > NAD(P)<sup>+</sup> + Nitrate + Hydrogen ion a menaquinol + Nitrate + Hydrogen ion > a menaquinone + Nitrite + Water + Hydrogen ion Nitrate + a ubiquinol > Nitrite + Water + a ubiquinone Nitrate + Hydrogen ion > Nitrite + Water 2 Nitric oxide + 2 Oxygen + NAD(P)H >2 Nitrate + NAD(P)(+) Nitrite + acceptor > Nitrate + reduced acceptor Nitric oxide + 2 Oxygen + NADH + NADPH <>2 Nitrate + NAD + NADP + Hydrogen ion Nitrate + Nitrate > Nitrate Nitrate + cytochrome c nitrite reductase + Nitrate <> Nitrite + cytochrome c nitrite reductase + Water + Nitrite | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways: | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| References | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Download (PDF) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in oxidoreductase activity
- Specific function:
- The nitrate reductase enzyme complex allows Pseudomonas aeruginosa to use nitrate as an electron acceptor during anaerobic growth. The alpha chain is the actual site of nitrate reduction
- Gene Name:
- narG
- Locus Tag:
- PA3875
- Molecular weight:
- 141 kDa
Reactions
| Nitrite + acceptor = nitrate + reduced acceptor. |
- General function:
- Involved in iron-sulfur cluster binding
- Specific function:
- The nitrate reductase enzyme complex allows Pseudomonas aeruginosa to use nitrate as an electron acceptor during anaerobic growth. The beta chain is an electron transfer unit containing four cysteine clusters involved in the formation of iron-sulfur centers. Electrons are transferred from the gamma chain to the molybdenum cofactor of the alpha subunit
- Gene Name:
- narH
- Locus Tag:
- PA3874
- Molecular weight:
- 58.1 kDa
Reactions
| Nitrite + acceptor = nitrate + reduced acceptor. |
- General function:
- Involved in nitrate reductase activity
- Specific function:
- The nitrate reductase enzyme complex allows Pseudomonas aeruginosa to use nitrate as an electron acceptor during anaerobic growth. The gamma chain is a membrane-embedded heme-iron unit resembling cytochrome b, which transfers electrons from quinones to the beta subunit
- Gene Name:
- narI
- Locus Tag:
- PA3872
- Molecular weight:
- 25 kDa
Reactions
| Nitrite + acceptor = nitrate + reduced acceptor. |
- General function:
- Involved in oxidoreductase activity
- Specific function:
- Catalytic subunit of the periplasmic nitrate reductase (NAP). Only expressed at high levels during aerobic growth. NapAB complex receives electrons from the membrane-anchored tetraheme protein napC, thus allowing electron flow between membrane and periplasm. Essential function for nitrate assimilation and may have a role in anaerobic metabolism
- Gene Name:
- napA
- Locus Tag:
- PA1174
- Molecular weight:
- 92.9 kDa
Reactions
| Nitrite + acceptor = nitrate + reduced acceptor. |
- General function:
- Energy production and conversion
- Specific function:
- Small subunit of the periplasmic nitrate reductase (NAP). Only expressed at high levels during aerobic growth. NapAB complex receives electrons from the membrane-anchored tetraheme napC protein, thus allowing electron flow between membrane and periplasm. Essential function for nitrate assimilation and may have a role in anaerobic metabolism
- Gene Name:
- napB
- Locus Tag:
- PA1173
- Molecular weight:
- 17.9 kDa
- General function:
- Involved in heme binding
- Specific function:
- Mediates electron flow from quinones to the napAB complex
- Gene Name:
- napC
- Locus Tag:
- PA1172
- Molecular weight:
- 22.7 kDa
- General function:
- Involved in unfolded protein binding
- Specific function:
- Chaperone required for proper molybdenum cofactor insertion and final assembly of the membrane-bound respiratory nitrate reductase 1. Required for the insertion of the molybdenum into the apo-NarG subunit, maybe by keeping NarG in an appropriate competent-open conformation for the molybdenum cofactor insertion to occur. NarJ maintains the apoNarGH complex in a soluble state. Upon insertion of the molybdenum cofactor, NarJ seems to dissociate from the activated soluble NarGH complex, before its association with the NarI subunit on the membrane
- Gene Name:
- narJ
- Locus Tag:
- PA3873
- Molecular weight:
- 27.3 kDa
Transporters
- General function:
- Involved in transmembrane transport
- Specific function:
- Involved in excretion of nitrite produced by the dissimilatory reduction of nitrate
- Gene Name:
- narK
- Locus Tag:
- PA3876
- Molecular weight:
- 50.6 kDa