|
Record Information |
|---|
| Version |
1.0 |
|---|
| Update Date |
1/22/2018 11:54:54 AM |
|---|
|
Metabolite ID | PAMDB000409 |
|---|
|
Identification |
|---|
| Name: |
Tetradecanoyl-CoA |
|---|
| Description: | Tetradecanoyl-CoA (or myristoyl-CoA) is an intermediate in fatty acid biosynthesis, fatty acid elongation and the beta oxidation of fatty acids. It is also used in the myristoylation of proteins. The first pass through the beta-oxidation process starts with the saturated fatty acid palmitoyl-CoA and produces myristoyl-CoA. A total of four enzymatic steps are required, starting with VLCAD CoA dehydrogenase (Very Long Chain) activity, followed by three enzymatic steps catalyzed by enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and ketoacyl-CoA thiolase. Myristoylation of proteins is also catalyzed by the presence of myristoyl-CoA along with Myristoyl-CoA:protein N-myristoyltransferase (NMT). Myristoylation is an irreversible, co-translational (during translation) protein modification found in animals, plants, fungi and viruses. In this protein modification a myristoyl group (derived from myristioyl CoA) is covalently attached via an amide bond to the alpha-amino group of an N-terminal amino acid of a nascent polypeptide. It is more common on glycine residues but also occurs on other amino acids. Myristoylation also occurs post-translationally, for example when previously internal glycine residues become exposed by caspase cleavage during apoptosis. |
|---|
|
Structure |
|
|---|
| Synonyms: | - Myristoyl-CoA
- Myristoyl-coenzyme A
- N-C14:0CoA
- N-C14:0Coenzyme A
- S-Tetradecanoyl-coenzyme A
- Tetradecanoyl CoA
- Tetradecanoyl Coenzyme A
- Tetradecanoyl-CoA
- Tetradecanoyl-CoA (N-C14:0CoA)
- Tetradecanoyl-coenzyme A
|
|---|
|
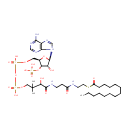
Chemical Formula: |
C35H62N7O17P3S |
|---|
| Average Molecular Weight: |
977.89 |
|---|
| Monoisotopic Molecular
Weight: |
977.313573819 |
|---|
| InChI Key: |
DUAFKXOFBZQTQE-XVDJLSDJSA-N |
|---|
| InChI: | InChI=1S/C35H62N7O17P3S/c1-4-5-6-7-8-9-10-11-12-13-14-15-26(44)63-19-18-37-25(43)16-17-38-33(47)30(46)35(2,3)21-56-62(53,54)59-61(51,52)55-20-24-29(58-60(48,49)50)28(45)34(57-24)42-23-41-27-31(36)39-22-40-32(27)42/h22-24,28-30,34,45-46H,4-21H2,1-3H3,(H,37,43)(H,38,47)(H,51,52)(H,53,54)(H2,36,39,40)(H2,48,49,50)/t24-,28-,29-,30?,34-/m1/s1 |
|---|
| CAS
number: |
3130-72-1 |
|---|
| IUPAC Name: | {[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({[hydroxy({3-hydroxy-2,2-dimethyl-3-[(2-{[2-(tetradecanoylsulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]propoxy})phosphoryl]oxy})phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid |
|---|
|
Traditional IUPAC Name: |
[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-4-hydroxy-2-[({hydroxy[hydroxy(3-hydroxy-2,2-dimethyl-3-[(2-{[2-(tetradecanoylsulfanyl)ethyl]carbamoyl}ethyl)carbamoyl]propoxy)phosphoryl]oxyphosphoryl}oxy)methyl]oxolan-3-yl]oxyphosphonic acid |
|---|
| SMILES: | CCCCCCCCCCCCCC(=O)SCCNC(=O)CCNC(=O)C(O)C(C)(C)COP(O)(=O)OP(O)(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP(O)(O)=O)N1C=NC2=C1N=CN=C2N |
|---|
|
Chemical Taxonomy |
|---|
|
Taxonomy Description | This compound belongs to the class of organic compounds known as 2,3,4-saturated fatty acyl coas. These are acyl-CoAs carrying a 2,3,4-saturated fatty acyl chain. |
|---|
|
Kingdom |
Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
|
Class |
Fatty Acyls |
|---|
| Sub Class | Fatty acyl thioesters |
|---|
|
Direct Parent |
2,3,4-saturated fatty acyl CoAs |
|---|
| Alternative Parents |
|
|---|
| Substituents |
- Coenzyme a or derivatives
- Purine ribonucleoside diphosphate
- Purine ribonucleoside 3',5'-bisphosphate
- N-glycosyl compound
- Glycosyl compound
- Beta amino acid or derivatives
- Organic pyrophosphate
- Monosaccharide phosphate
- 6-aminopurine
- Purine
- Imidazopyrimidine
- Monoalkyl phosphate
- Aminopyrimidine
- Imidolactam
- Alkyl phosphate
- Pyrimidine
- Primary aromatic amine
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Organic phosphate
- N-substituted imidazole
- N-acyl-amine
- Monosaccharide
- Fatty amide
- Saccharide
- Heteroaromatic compound
- Oxolane
- Imidazole
- Azole
- Thiocarboxylic acid ester
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxamide group
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Sulfenyl compound
- Thioether
- Thiocarboxylic acid or derivatives
- Carboxylic acid derivative
- Carboxylic acid amide
- Hydrocarbon derivative
- Primary amine
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Amine
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework |
Aromatic heteropolycyclic compounds |
|---|
| External Descriptors |
|
|---|
|
Physical Properties |
|---|
| State: |
Solid |
|---|
| Charge: | -4 |
|---|
|
Melting point: |
Not Available |
|---|
| Experimental Properties: |
|
|---|
| Predicted Properties |
|
|---|
|
Biological Properties |
|---|
| Cellular Locations: |
Cytoplasm |
|---|
| Reactions: | |
|---|
|
Pathways: |
|
|---|
|
Spectra |
|---|
| Spectra: |
|
|---|
|
References |
|---|
| References: |
- Hunt MC, Ruiter J, Mooyer P, van Roermond CW, Ofman R, Ijlst L, Wanders RJ: Identification of fatty acid oxidation disorder patients with lowered acyl-CoA thioesterase activity in human skin fibroblasts. Eur J Clin Invest. 2005 Jan;35(1):38-46. Pubmed: 15638818
- Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
|
|---|
| Synthesis Reference: |
Not Available |
|---|
| Material Safety Data Sheet (MSDS) |
Not Available |
|---|
|
Links |
|---|
| External Links: |
|
|---|